硼碳氮氧化物作为一种新型无金属光催化剂
摘要
硼基纳米材料作为无毒、地球上丰富的(光)电催化剂材料正在成为太阳能转换中用于生产太阳能氢燃料和环境修复的材料。硼氧氮化碳 (BCNO) 是一种四元半导体,具有电子、光学和物理化学特性,可以通过改变硼、氮、碳和氧的组成进行调节。然而,BCNO 的结构和光催化活性之间的关系还有待探索。我们进行了深入的光谱分析,以阐明使用两种不同的氮前体的影响以及退火温度对 BCNO 制备的影响。 BCNO 纳米盘 (D =6.7 ± 1.1 nm) 具有乱层氮化硼衍射图案,使用盐酸胍作为氮源前体,在 800°C 下热退火后制备。 BCNO 纳米盘的 X 射线光电子能谱 (XPS) 表面元素分析显示 B、C、N 和 O 的组成分别为 40.6%、7.95%、37.7% 和 13.8%。根据固态 11 B NMR分析,盐酸胍衍生的BCNO纳米盘显示形成了各种三配位BNx (OH)3-x 物种,这也是光催化活性位点之一。 XRD 和深入的光谱分析证实了 BCNO 掺杂的六方氮化硼纳米盘的制备。相比之下,BCNO 使用三聚氰胺作为氮前体在 600°C 下退火,由层状纳米片组成,这些纳米片由 B、C、N 和 O 原子以蜂窝晶格共价键合组成,XRD、XPS 和固态 NMR 证明了这一点分析 ( 11 B 和 13 C) 分析。三聚氰胺衍生的 BCNO 层状结构的 XPS 表面元素组成由高碳成分 (75.1%) 和相对较低的硼 (5.24%) 和氮 (7.27%) 成分组成,这表明形成了 BCNO 掺杂的氧化石墨烯分层片状结构。发现这一系列三聚氰胺衍生的 BCNO 掺杂的氧化石墨烯层状结构表现出最高的光催化活性,超过了石墨氮化碳的光催化活性。在这种层状结构中,四配位BNx的形成 (OH)3-x (CO) 物种和丰富的石墨域被提议在 BCNO 掺杂的氧化石墨烯层状结构的光催化活性中发挥重要作用。对于 BCNO 掺杂的六方氮化硼纳米盘和 BCNO 掺杂的氧化石墨烯层状结构,测得的光学带隙能量分别为 5.7 eV 和 4.2 eV。最后,BCNO 表现出超长光致发光,BGH01、BGH03、BMH01、BMH03 的平均衰减寿命分别为 1.58、2.10、5.18 和 8.14 µs。该研究提供了一种新型的无金属光催化体系,并首次对BCNO基光催化剂的来源进行了结构分析。
图形摘要
<图片>
介绍
无金属纳米材料正在成为具有高结构和化学稳定性的经济高效、对地球友好的(光)催化剂,适用于各种应用,包括太阳能燃料生产、环境修复、二氧化碳减少、有害微生物的消毒以及有机物的选择性化学合成。化合物 [1,2,3,4,5,6,7]。与其金属对应物相比,不含金属的催化剂也不易中毒并导致更长的循环寿命。因此,寻找和开发稳定、高效且具有成本效益的光催化剂残留物的新材料是至关重要且具有挑战性的研究工作。碳基材料如石墨氮化碳 (CN) [4, 8, 9]、碳点 (C-dot) [2, 3, 10] 和石墨烯基材料 [7, 11] 已被广泛研究由于其优异的物理化学性质、结构和化学稳定性,以及易于从地球上丰富的元素合成。最近,硼基(光)催化剂已被开发为具有卓越性能的无金属光催化体系。值得注意的是,以其硬度着称的碳化硼显示出无金属可见光光催化产氢,超过了最先进的碳基 CN 光催化剂 [12, 13]。高表面积碳掺杂六方氮化硼 (BCN) 纳米片对 H2 和 O2 生成以及 CO2 还原和捕获表现出可见光光催化活性,为光系统带来了新的可能性 [14, 15]。其他含硼(光)电催化剂,如氧氮化硼 (BNO)[16, 17]、磷化硼 (BP) [18, 19]、掺硼石墨烯 [20]、碳氮化硼 (BCN) [14]、掺硼氮化碳(B-掺杂CN)[21]和元素硼[22, 23]已表现出显着的(光)电催化活性[13]。
硼碳氮氧化物 (BCNO) 是一种硼基纳米材料,与其他材料相比,研究较少。它最初是在其 BCN 前身之后开发的,一种带隙 约为 2 eV 的半导体,用于替代基于氧氮化物和氮化物化合物的有毒磷光体 [24, 25]。 B、C、O 和 N 原子取代石墨烯或六方氮化硼 (hBN) 网络产生了具有可调光致发光特性和带隙范围从 0 eV(石墨烯)到 5.9 eV(hBN)的 BCNO 化合物 [26] .这些理想的半导体和光致发光特性最近吸引了研究人员开发一种新的合成方法来合成具有更高结晶度 [27]、可控形状 [28] 和原子级薄二维结构 [29] 的低维 BCNO 纳米结构。以前的工作研究了退火温度和时间对调节 BCNO 光致发光特性的影响,但没有提供深入的结构表征 [30, 31]。在本文中,我们研究了使用不同氮源前体、煅烧温度(800°C 对 600C)和煅烧时间(0.5 小时对 12 小时)对 BCNO 纳米结构的结构光催化活性的影响。
方法
化学品和仪器
硼酸 99.99% (H3BO3)、三聚氰胺 99% (C3H6N6) 和六亚甲基四胺 ≥ 99% (C6H12N4) 购自 Alfa Aesar,无需进一步纯化即可使用。盐酸胍 99.5% (CH5N3 HCl) 购自 Arcos Organics。 BCNO 是根据文献通过低温退火法合成的 [25, 32]。 UPS 分析在 ULVAC-PHI PHI 5000 Versaprobe II 中进行,使用 He I 21.22 eV 作为具有 5 V 偏压的光子源。通过透射电子显微镜(JEOL,JEM-ARM200FTH)分析 BCNO 样品的形态。使用 Bruker D2 光谱仪获得 XRD 衍射。使用光致发光光谱仪(PerkinElmer,LS55)获得溶液中的光致发光发射光谱,溶液中 BCNO 的光吸收光谱由紫外可见分光光度计(HITACHI,U-3900)测定。 X 射线光电子能谱通过高分辨率 X 射线光电子能谱仪(ULVAC-PHI,PHI Quantera II)使用 Al Ka X 射线作为激发源进行分析。将 BCNO 溶液滴铸到硅基板上用于 XPS 表征。结合能以 284.8 eV 校准到碳。 XPS 峰解卷积和拟合使用 CACS XPS 软件进行。绝对 PLQY 根据文献 [33] 进行,并由 CCD 相机(PIXIS 256BR,普林斯顿仪器)检测。绝对 PLQY 的测量是通过使用基于光纤的光谱仪进行的,包括校准积分球系统 (Labsphere) 和电荷耦合器件 (CCD) 相机(PIXIS 256BR,普林斯顿仪器)。二极管激光器 (λ =375 nm,Becker &Hickl GmbH) 用作泵浦源。通过使用脉冲氮激光 (λ) 在正面配置中测量时间分辨光致发光 =337.1 nm,LTB Lasertechnik Berlin GmbH)作为激发源,由数字延迟发生器(DG645,Stanford Research Systems)触发。信号由光子计数光电倍增管(PMC-100-1,Becker &Hickl GmbH)检测,光子计数由多尺度模块(MSA-300,Becker &Hickl GmbH)累积。红外光谱由傅立叶变换红外光谱仪(Bruker,Vertex 80v)使用衰减全反射(ATR)记录。使用配备 9.4 T 磁体的 Bruker Avance III 400 NMR 光谱仪使用 4 mm 魔角旋转 (MAS) 探针获得核磁共振谱。 11 B MAS NMR 使用自旋回波方法以 10 kHz 的自旋速率记录。以 4 秒的循环延迟收集了 8000 次扫描。化学位移参考 19.6 ppm 的 1 M H3BO3 水溶液。 13 C CP/MAS NMR 光谱是使用交叉极化 (CP) 序列以 12.5 kHz 的旋转速率记录的。以 4 秒的循环延迟收集了 30,000 次扫描。所有 13 C 化学位移参考纯三甲基硅烷,使用 38.48 ppm 的金刚烷 CH2 峰的二级参考。
BCNO的准备
在这项研究中,使用两种不同的氮前体源制备了两个系列的 BCNO,同时固定硼和碳前体源、前体比率、退火温度和时间。通过固定本研究中制备的每个系列 BCNO 的所有其他反应参数,还研究了退火温度和时间的影响。简而言之,将预定摩尔比的所有三种前体组分加入蒸馏水中并加热至 90°C,直到溶液看起来均匀(表 1)。将粘性混合物在烘箱中干燥过夜,得到干燥的白色固体。使用研钵和研杵将白色固体研磨成细粉。固体前体在炉中在预定温度和时间下煅烧,如表 1 所示,在环境大气压下以 5°C/分钟的升温速率。将微黄色粉末试样在炉内自然冷却至室温后研磨成细粉。
BCNO的净化策略
通过在水和乙醇中以 6000 rpm 离心 10 分钟(1:10 v/v,10 mg/mL 浓度)来纯化制备的 BCNO。离心后,将产物重新溶解在蒸馏水中,用乙醇以 1:10 v/v 的水:乙醇比稀释。纯化的 BCNO 沉积在碳涂层铜网上用于 TEM 分析。为了制备 SEM 和 XPS 样品,将纯化的 BCNO 样品滴铸到硅片上。在样品沉积之前,通过在每种溶剂中用水、丙醇和丙酮超声 10 分钟来清洁硅晶片。 UPS 样品的制备与针对 SEM 和 XPS 样品所述的程序类似,不同之处在于样品沉积在氧化铟锡 (ITO) 涂层玻璃上。
大块氮化碳 (CN) 的制备
散装 CN 是通过文献 [34] 中报道的简便方法合成的。简而言之,将三聚氰胺粉末放入坩埚中,在常压、550℃、5℃/min的升温速率下退火4小时。
光催化染料降解程序
通过亚甲蓝 (MB) 光降解作为模型反应来评估各种 BCNO 样品的光催化活性。在典型的染料降解实验中,将 10 毫克 BCNO 样品加入含有 15 毫升 MB 溶液 (10 ppm) 的样品瓶中。在黑暗中搅拌 10 分钟后,用 100 瓦氙灯(250 纳米 ~ 1100 纳米)照射样品瓶。使用移液管以 20 分钟的时间间隔从溶液中提取动力学样品 (2 mL),直到总光催化降解时间达到 80 分钟。通过紫外-可见光谱仪分析不同时间间隔的动力学样品。 MB浓度的变化是根据比尔定律得出的。
结果与讨论
BCNO的合成
在我们制备具有高结晶度的低维 BCNO 纳米结构以促进电荷传输的研究中,我们发现 BCNO 具有明显不同的化学结构和光催化活性。基于文献 [25, 32] 中报道的 BCNO 合成,我们研究了使用两种不同氮前体来源的影响以及热退火温度和时间对 BCNO 结构-性质演变的影响。在本研究中,分别使用硼酸和六亚甲基四胺作为硼源和碳源制备了两个系列的 BCNO(表 1)。 BCNO 使用三聚氰胺和盐酸胍作为氮源合成,分别表示为 BMH 和 BGH。在系统地研究了每个反应条件后,BMH 系列仅在 600°C 的较低温度下退火 12 小时时才表现出光催化活性。 BGH 系列仅在 800°C 高温退火 12 小时时表现出光催化活性。在这两个系列中,硼酸、三聚氰胺和盐酸胍的摩尔比固定为 3:1,而六亚甲基四胺的摩尔比从 0.1 到 0.3 不等。这些六亚甲基四胺前体的比率在样品名称中分别表示为 BMHH01/BGH01 和 BMH03/BGH03(表 1)。
图 1 显示了 BGH 在 800°C 下煅烧 12 小时的代表性 TEM 图像,由具有准球形纳米颗粒形态的结晶核和 D 组成 =6.7 ± 1nm。所制备的 BGH 很容易分散在水中(附加文件 1:图 S1),并且由离散纳米粒子在 TEM 铜网格上的沉积所证明。 BGH 和 BMH 的水分散性很可能源于基于 BGH 和 BMH 带负电荷的表面电位的静电稳定。固态 11 B NMR 分析表明,BMH 和 BGH 纳米结构中的大量羟基证实了它们在水性介质中的分散性。在高放大倍数下,每个准球形纳米颗粒表现出独特的 (002) 平面晶格间距,测量为 0.338 nm,这与文献报道一致 [24, 35](图 1c)。与通过在共晶盐环境中热退火获得的 5 nm BCNO 纳米颗粒相比,本工作制备的 BGH 纳米颗粒具有高结晶度 [25](图 1)。接下来,我们表征了基于文献报告使用硼酸、三聚氰胺和六亚甲基四胺制备并在 600°C 下退火 12 小时的 BMH。与 BGH 的形态相反,BMH 由形状不明确的多层片组成(图 1)。在更高的放大倍数下,多层片材边缘的 TEM 图像(图 1d)显示具有结构变形特征的纳米片 [36],如图 1e 所示。图 1f 显示了 BMH 的代表性 SEM 图像,包括无特征的微米级聚集体。然而,与 BGH 系列不同的是,使用三聚氰胺作为氮源前体在 800°C 的高温煅烧不会产生纳米盘形态。在 ESI 中可以获得在不同反应条件下合成的其他 BGH 和 BMH 系列化合物的 TEM、XRD、UV 吸光度和光致发光。
<图片>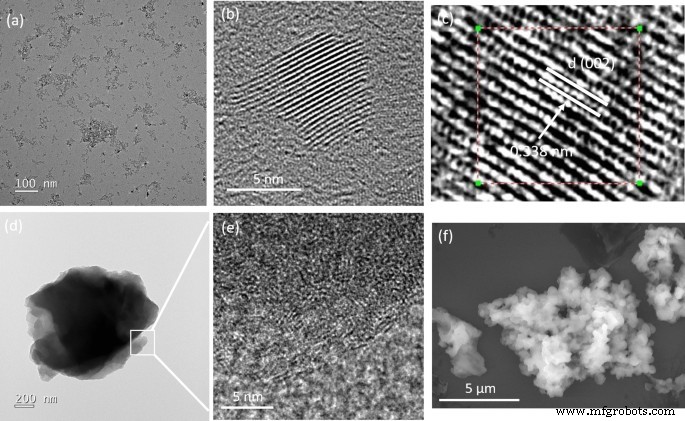
从稀乙醇溶液中沉积的制备的 BGH 和 BMH 的代表性透射电子显微照片 (TEM) 和扫描电子显微照片 (SEM)。 一 , b BGH的高低倍透射电镜,c BGH 的高分辨率 TEM,具有不同的 (002) 晶格间距 0.338 nm,d 低倍率下代表性 BMH 的 TEM 图像,e 图 1d 中方框区域的放大 TEM 图像 显示层状氧化石墨烯内结构变形的特征,以及 f BMH的代表性SEM图像
图 2 显示了我们实验室在表 1 中列出的规定反应条件下制备的 BMH03(绿色迹线)、BMH01(红色迹线)和 BGH01(蓝色迹线)的 XRD 谱。BGH01 表现出以 26.6° 和43.1° (2θ),这是乱层氮化硼 (t-BN) 的特征衍射图。以 26.6° 为中心的宽峰源自 (002) 反射面,43.1° 宽峰对应于由六方氮化硼 (h-BN) 诱导的 (10) 反射面 [37]。 BMH 的 XRD 图案主要由 2θ 约 25.4° 和 42.4° 处的两个宽衍射图案主导,它们分别是石墨六方晶体结构的 (002) 和 (10) 带的特征图案 [38]。通常表示的 (10) 带也与乱层碳的 2D 反射有关 [39]。此外,由于在石墨烯或氧化石墨的结构中掺入了掺杂剂或杂质,因此在 2θ 周围 10.9°处没有峰,而在 约 25.4°处出现宽带[40,41,42,43, 44,45]。因此,提出BMH的主要结构是掺杂的氧化石墨烯是合理的。
<图片>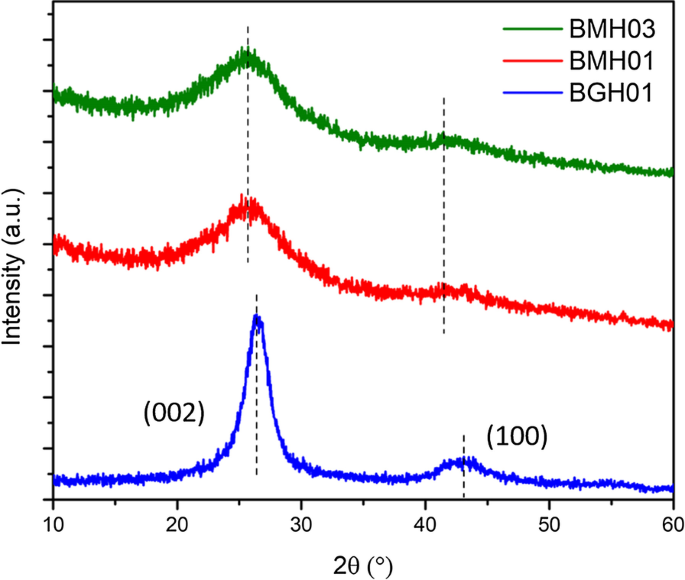
多晶 BMH03(绿色迹线)、BMH01(红色迹线)、BGH01(蓝色迹线)和 BGH01 的 XRD 图案。大约 BGH01 系列的宽峰。 26.6° 代表 h-BN 的 (002) 平面反射和另一个宽峰。 43.1° 表示 h-BN 的未解析反射面。 BMH 的衍射图案显示出两个广泛的反射,中心位于 ~ 25.4° 和 ~ 42.4°。其他 BCNO 系列的 XRD 模式显示在附加文件 1:图 S3)
BGH(胍系列)结构分析
执行 XPS、FTIR 和固态 NMR 光谱以更深入地了解 BGH 的分子结构。 XPS 用于确认 B、C、N 和 O 元素的核心能级电子的存在以及它们各自在 BGH 化合物中的化学键合。图 3a-e 显示了 BGH 纳米盘的典型 XPS 光谱。根据 XPS 表面元素组成分析,BGH 的 B 和 N 含量较高(各约 40%),C 和 O 的含量较低,分别为 约 8% 和 13%(附加文件 1:表 S3)。 B 和 N 组成的 接近 1:1 化学计量与 XRD 分析相称,这证实了我们实验室制备的 BGH01 由涡层氮化硼结构组成。所有 XPS 光谱都拟合了具有 R 的高斯函数 2 > 0.99,由每个元素的 XPS 光谱中的红色和绿色曲线表示。对于 BGH 系列,B1s 光谱被解卷积为两条拟合曲线,对应于 B-O 键合(189.7 eV 结合能)和 B-N 键合(190.7 eV 结合能)。 N1s 光谱拟合有两条高斯曲线,由结合能为 397.3 eV 的 N-B 键和结合能为 398 eV 的 B-N-O 键组成 [46]。 N-B 和 B-N-O 键合都表明存在 O 掺杂的 hBN 或形成了 BNO 化合物 [47]。由于 BGH 的合成是在大气条件下进行的,如文献 [47] 中所报道,氧原子也被纳入 h-BN 结构域以进行 B-O 键合。通过分析 O1s 光谱证实了这一假设,该光谱显示对应于 B-O 键合的单峰(结合能为 532 eV)。这一结果进一步支持了我们最初的假设,即 BGH 系列由 O 掺杂的 hBN 组成,并且大部分氧原子与硼原子键合。 BGH 光谱的 C1 被解卷积为三个 C 物质,即 C-O、C-B 和 C-O-C,它们分别以 283.7 eV、285 eV 和 287 eV 键合。由于 BGH 系列中 C 的低组成和 B 和 N 的 1:1 化学计量比,我们进一步推测 BGH01 的结构是 C 和 O 掺杂的 h-BN。如 XPS 光谱所示,所提出的结构得到了 hBN 域内 C-B、B-O 和 B-N-O 键合的支持(图 3)。因此,将在 800°C 下制备的 BGH 纳米盘的结构推断为 C 和 O 掺杂的 hBN 是合理的。此外,粉末 XRD 和高分辨率 TEM 支持在蜂窝晶格中形成共价键合的 B、C、N 和 O,其晶格间距类似于乱层氮化硼的晶格间距。在整个手稿中,BGH01 的结构将被称为 BCNO 掺杂的 hBN。
<图片>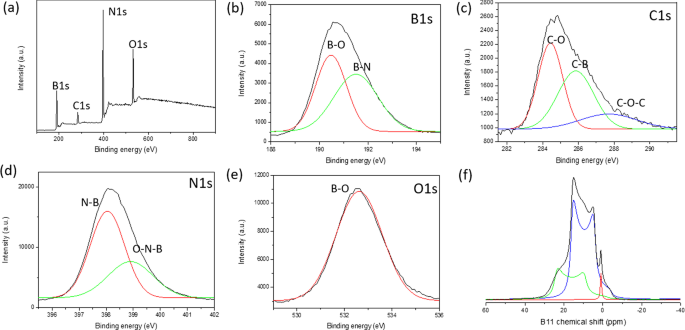
一 BGH01 的 XPS 测量光谱。 b 的核心能级光谱 B1s,c C1s,d N1s 和 e O1。每个解卷积峰都拟合有高斯函数。 f 11 BGH01的固态MAS NMR谱。 ( 11 BGH01-LT的B固态MAS NMR见附加文件1:图S10)
我们还研究了煅烧温度和时间对 BCNO 纳米结构的结构光催化活性的影响,同时保持其他合成参数不变。在提高反应温度(从 600°C 到 800°C)并将反应时间从 30 分钟增加到 12 小时后,B-N 和 B-O 键合总体增加(附加文件 1:图 S6)。相比之下,B-C 键随着反应温度和时间的增加而减少,这意味着在牺牲亚稳态 B-C 键的同时,会形成能量稳定的六方 B-N 和 B-O 键 [48]。正如预期的那样,B-C 键合组成随着六亚甲基四胺前体(作为 C 源)的比例增加而增加,同时保持其他参数不变 [49]。 ESI 中提供了在不同煅烧温度、时间和前体比率下制备的 BGH 化学键演变的更详细趋势(附加文件 1:图 S6 和 S7)。
1 11 B 固态 MAS NMR 用于定量分析 BGH 和 BMH 中每个 B 相关的键组成。在此,我们首次利用固态 MAS 13 在分子水平上对 BCNO 进行了详细的结构表征 C 和 11 B NMR 收集特定的 C 和 B 相关键合。尽管 hBN 纳米材料及其缺陷引起的电子特性引起了人们极大的兴趣,但 hBN 边缘的分子结构和缺陷结构在很大程度上是未知的 [50]。硼相关纳米材料的结构表征缺乏是由于难以分析固态 11 B NMR谱因为 11 B 是半整数四极原子核 (I =3/2) [51, 52]。固态 11 由于二阶四极耦合导致信号失真,B NMR 也难以解释,只能通过 MAS NMR 进行部分平均 [53]。此外, 11 的化学位移范围 B NMR 相对较窄,这使得各种硼种类的宽峰、重叠峰和扭曲峰的峰归属极具挑战性[54]。在这项研究中,我们执行了 11 B 固态 NMR 在 9.4 T 下记录,并且使用顶旋实线形状分析 (SOLA) 对光谱进行解卷积。遵循 CP-MAS 11 在文献报道的 B NMR 实验中,我们通过考虑四极耦合常数 (C Q) 和电场梯度 (EFG) 张量不对称性 (η 问) [51, 52, 55]。图 3f 显示了固态 11 BGH的B自旋回波核磁共振谱具有三个主峰和δ iso 分别以 28.3 ppm、20 ppm 和 1.2 ppm 为中心。基于 CP-MAS 11 的文献研究 氮化硼及其相关结构的B NMR,峰具有δ iso 为 28.3 ppm(绿色曲线拟合)和 C 2.85 MHz 的 Q 对应于具有单个羟基的三角平面 BN2(OH) 物质 [53,54,55]。当羟基或氧桥原子取代了三角平面硼位点周围的氮原子时,一个具有 δ 的新 B 物种 出现了 20 ppm 的 iso(蓝色曲线拟合)。这种较低的化学位移信号很可能归因于另一个具有两个羟基或一个羟基和一个桥接氧原子的三角硼位点(BN(OH)2 或 BNO(OH) 位点)。 δ处的尖峰 1.2 ppm 的 iso(红色迹线拟合)对应于四坐标四面体 B 位点,可能由氮和多个羟基或桥接氧原子 (52-54) 或碳相关的 C-B 键协调 [46, 52]。 CP-MAS 11 BGH01 的 B NMR 分析揭示了与 XPS 和 XRD 分析相称的各种 B-N、O-B 和 B-C 键合。此外,固态 11 B NMR 还显示,大多数硼与氮和一个或多个羟基键合,作为 BNx (OH)3-x 物种。推测这些羟基化物质通过氢键和静电稳定在水溶液中提供胶体稳定性。我们还观察到四配位 B 物种如 BNx 的减少 (OH)4-x 或 BNx (O)(OH)3−x (其中 O 是桥接氧)和硼醇环 [54, 56] 随着反应温度的升高。同时,各种三坐标BNx (OH)3-x 当退火温度从 600°C 增加到 800°C 时出现了物种(附加文件 1:图 S8 和 S10)。该结果表明,在高温下,硼醇环与氨反应形成各种羟基化的 BNx (OH)3-x 或 BNx (O)(OH)2-x 物种 [55](附加文件 1:图 S10)。
BMH 的结构分析
基于我们通过 XPS 和 FTIR 以及 11 进行的有力结构分析 B、 13 C 固态 MAS NMR,BMH 的结构被认为是 BCNO 掺杂的氧化石墨烯。根据 XPS 表面元素分析,BMH 由 75% 的碳物种和仅 5% 的硼相关物种组成(附加文件 1:表 S3)。解卷积的 B1s 光谱显示 BCN 和 BN 键合分别为 191.7 和 192.4 eV 结合能(图 4)。 C1s 物种显示出两种不同的光电发射信号,对应于 C-C 键合(sp 2 和 sp 3 C-C 键),结合能约为 285.0 eV,较弱的成分来自出现在 285.6 eV 的 B-C-N 键 [57]。 288.0 eV 和 288.7 eV 处的其他相对较小的信号分别是由于 C-N3 和 C =O 键合 [46, 57, 58]。据推测,氧化的 C 原子(C =O)在氧化石墨烯域的边缘形成,C-N3 键是 CN 的特征峰。以 399.0 eV 结合能为中心的 N1s XPS 光谱可以拟合两条高斯曲线,由 399.2 eV 结合处的 C-N-B 和 400.0 eV 处的 C =N 结合组成 [46, 57, 59, 60]。根据 XPS 分析,BMH 系列仅包含约 10-25% 的 O 相关键合,而 BGH 系列中的 O 相关键合 大约 35%。以 531.2 eV 结合能为中心的 O1s 光谱可以解卷积为 C =O 和 CO 键合,这可能归因于氧化石墨烯域。 13 C 固态 CP-MAS NMR 进一步显示在 40.2 ppm 处存在石墨 C =C 键合,这与 XRD 和 XPS 结果 [38](图 5)相符。在本文报道的每个 BMH 系列中, 13 C NMR 显示在 160 和 154 ppm 对应于 C 的碳物种的两个等摩尔比 α 和 C 分别为 β,通常在石墨氮化碳结构中发现 [61](图 5 和附加文件 1:表 S4)。与根据文献程序合成的本体 CN 相比 [34],C 的特征共振的化学位移 α 和 C β 峰分别出现在 164 和 156 ppm(图 S13)。硼掺杂到 CN 庚嗪结构中可能导致 BMH 系列和块状 CN 之间存在这些轻微的化学位移差异(附加文件 1:图 S13)。
<图片>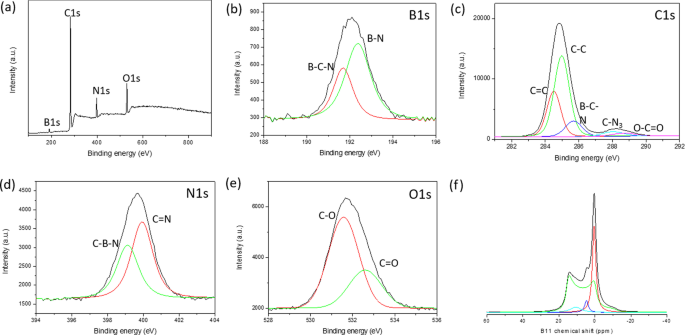
a Survey XPS spectra of BMH01 and 11 B NMR spectra. Core level spectra of b B1s, c C1s, d N1s, and e O1s. Each core spectra were fitted with a black trace, while the red and green traces under the peak were deconvoluted using a Gaussian function. f 11 B solid-state MAS NMR were deconvoluted using SOLA analysis to tricoordinate and tetracoordinate B-sites. The XPS and 11 B solid-state MAS NMR of other BMH series compounds can be found in Additional file 1:Figure S7, S11, and S12)
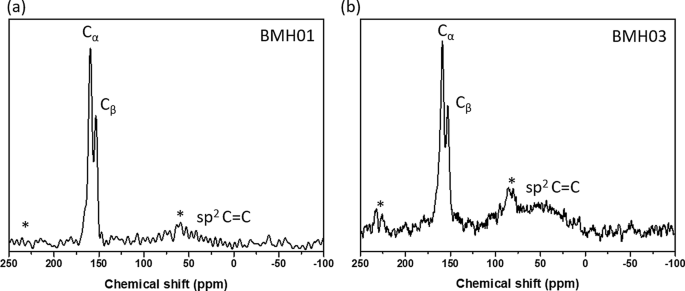
Solid-state 13 C MAS NMR of a BMH01 and b BMH03
Due to the abundant evidence of the presence of carbon nitride (CN) in the BMH series based on solid-state NMR spectroscopy (Fig. 5), we considered three possibilities of interactions between BCNO-doped graphene oxide and CN in BMH, namely:(i) bulk phase separation, (ii) disordered 2D network, and (iii) layered intercalation [62]. To eliminate the possibility of bulk phase separation, we examined the XRD pattern of CN nitride andBMH as well as a physical mixture of both in a 1:1 mass ratio. We found that the XRD patterns of the mixtures showed only the diffraction pattern of the bulk CN with a slight reduction in the crystallinity compared to the pristine CN diffraction [63] (Additional file 1:Fig S4). Since the XRD pattern of BMH did not possess any diffraction peaks that corresponded to CN, this experimental result confirmed that CN did not form as a bulk-separated domain during the synthesis of BMH (Additional file 1:Fig. S4). We also considered the formation of a disordered 2D network, in which CN and the doped-graphene oxides are bonded on the same 2D plane [62]. Based on a literature report on a CN/graphene oxide 2D matrix, the XRD pattern of a disordered 2D network showed characteristic peaks for both species with a slight peak broadening and a slight peak shifting [64]. However, the XRD pattern of BMH (Fig. 2) did not contain any signature diffractions of CN. A previous study also showed that characteristic peaks of graphene oxide disappeared in a graphitic CN/amorphous CN/graphene oxide composite due to the layer-by-layer interactions [65]. Therefore, it is reasonable to propose that CN is intercalated between the doped-graphene oxide layers. The FTIR spectrum of the selected BGH and BMH series is shown in Additional file 1:Fig. S5.
Boron-related bonding within the BMH series was investigated using 11 B solid-state MAS NMR at 9.4T, and the broad NMR spectrum was deconvoluted using the SOLA analysis showing the presence of both tricoordinate and tetracoordinate boron site. The SOLA analysis yielded four line fittings under the broad 11 B NMR spectrum, which could be assigned as trigonal planar BN2(OH) or BN2O at a δiso of 19.8 ppm and a CQ of 2.85 MHz (green fitting). The bay and corner B sites as in B-doped CN appeared at a δ iso of 5 ppm and δ iso of 11 ppm, respectively [61]. These assignments are also commensurate with the formation of CN based on the 13 C NMR and XPS analyses. Compared to the BGH series, the relative composition of the tetracoordinate B(IV) site of BMH was much higher (ca. 55% in the BMH series vs. 3% in the BGH series). However, the tetracoordinate B(IV) species in BMH appeared at a lower chemical shift than those found in BGH (Fig. 3f) and was therefore presumed to be the BN2(OC)2−x (OH)x species [46]. Notably, the h-BN domain was absent from the BMH series prepared via thermal annealing at a lower temperature (600 °C). However, upon increasing the thermal annealing temperature from 600 °C to 800 °C, the structures of BMH01HT-30 min and BMH01HT-12 h showed a high composition of tetracoordinate BN2(OH)2 species and the tricoordinate BN3 bonding (Additional file 1:Fig. S11 and S12). The presence of a high composition of tetracoordinate BN2(OH)2 and BN3 bonding was shared among all the inactive BCNO investigated in this study. Moreover, although BMH01HT-12 h possessed an identical surface elemental composition to that of BGH01, the solid-state 11 B NMR revealed that both compounds possessed significant structural differences, which explained for their differences in photocatalytic activity (Additional file 1:Table S3 and Fig. S12).
In light of the moderate photocatalytic activity of BMH01 and BGH01 (Fig. 7), further synthesis optimization was performed to expand their light absorption spectrum into the visible light region. Previous literature showed that increasing the composition of the hexamethylenetetramine precursor (as a carbon source) could modulate the bandgap and photoluminescence properties of BCNO. Based on these reports, BMH and BGH compounds with a higher ratio of hexamethylenetetramine were prepared accordingly while keeping the other parameters constant. The optimized BCNO with a higher carbon content is denoted as BMH03 and BGH03, in which the molar ratio of carbon source was increased from 0.1 to 0.3. The higher ratio of hexamethylenetetramine precursor yielded BMH03 with a higher composition of the graphitic domain as in sp 2 C = C, and a small peak emerged which corresponded to BCN bonding at 191 eV binding energy (Additional file 1:Fig. S7). The increase in the graphitic sp 2 C = C domain upon increasing the concentration of hexamethylenetetramine is consistent with the role of hexamethylenetetramine as both a C and N source in the synthesis of N-doped graphite [66]. The increased sp 2 C = C graphite bonding in BMH03 was further confirmed via 13 C CP-MAS NMR with the emergence of a more prominent peak centered at 40.2 ppm, which is the signature of graphitic C = C(H) bonding (Additional file 1:Table S4).
Optical Properties
The optical properties of BMH and BGH were investigated using UV–visible absorption and photoluminescence spectroscopy, as shown in Fig. 6. Since BGH and BMH series possessed distinctively different structures, and the optical properties of nanomaterials are highly correlated with their structures, the origin of absorption and luminescence for both series were also found to be different. In this study, BGH01 quasi-spherical nanoparticles showed a featureless UV–vis spectrum. (Fig. 6a, red trace). As the ratio of hexamethylenetetramine increased, the intensity of absorbance peaked at 237 nm for BGH03 and increased with the emergence of an additional broad absorption peak centered around 330 nm (Fig. 6a, blue trace). The origin of the optical properties of BCNO is controversial due to the lack of structural analysis of BCNO nanomaterials. The most widely cited origin of photoluminescence of BCNO is attributed to the formation of B, C, N, and O self-interstitial sites, substitutional impurities, and native point defects within hBN or the graphene matrix [50, 67, 68]. Other possible photoluminescence mechanisms in the BCNO system include the electronic transition from the nitrogen-vacancy (VN) levels to the carbon impurity levels, the electronic transition between the closed-shell BO − and BO 2− anions, and intrinsic state emission and defect state emission (surface energy traps). The featureless UV absorbance of BGH01 can be attributed to the electronic transition between the valence band (VB) and conduction band (CB) of boron nitride with a bandgap energy of ca. 5.9 eV. The lower energy absorption in BGH03 was a result of the mid-gap absorption from the valence band to the nitrogen-vacancy (VN) level located approximately 0.7–1.0 eV below the conduction band of hBN.(62) The absorption peak at ca. 330 nm could be attributed to the presence of C-related impurities level located ca. 2–4 eV below the conduction band of hBN [69,70,71]. Under 365 nm excitation, BGH01 produced a broad emission with three bumps located at 412 nm, 445 nm, and 489 nm, respectively. Based on the BGH structural analysis, in which the dominant structure was composed of BN and BO-related bonding, the photoluminescence of the BGH series could be most likely originated from the B-O luminescence centers [24, 47, 72]. The yellow-green emission at 445 and 489 nm could be induced by the transition from the VN level to the carbon-related and oxygen defect levels (2–4 eV) below the conduction band of h-BN [69,70,71]. As the C composition increased in BGH03, the emission wavelength was further red-shifted to 506 nm, consistent with literature reports [73, 74]. The stacked photoluminescence (PL), UV absorbance, and Tauc plot for different BGH series prepared in this study are presented in Additional file 1:Fig. S14.
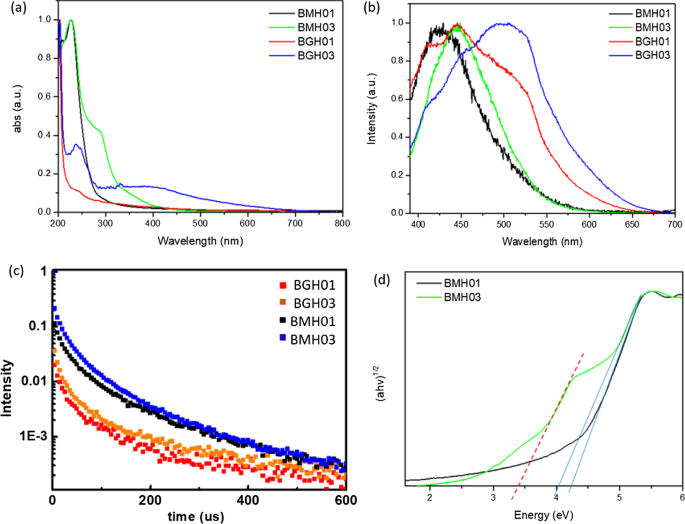
a Overlay UV absorbance spectra of BMH01, BMH03, BGH01, and BGH03. b Stacked photoluminescence spectra of BMH01, BMH03, BGH01, and BGH03 upon excitation at 365 nm c stacked photoluminescence lifetime decay spectrum of BMH01, BMH03, BGH01, and BGH03 monitored at a predetermined λ max for each sample. Samples were excited with 337 nm laser pulses at 298 K, d Overlay Tauc plot (αhv) 1/2 vs. hv, for BMH01 (black trace) and BMH03 (green trace). The optical band gap is represented by thesolid line, while the interband state for BMH03 is represented by the red dashed line
Based on XRD and various spectroscopic analyses, the structure of BMH was deduced to be dominated by BCNO-doped graphene oxides (Fig. 4, Additional file 1:Fig. S7, and Fig. S12, and Table S4). Thus, the optical properties of the BMH series are hypothesized to be more closely related to the carbon-quantum dot (CD) [75, 76] and doped-graphene oxides systems [44, 77,78,79]. Based on the origin of photoluminescence of the CD and graphene oxides, the optical properties of BMH prepared in this study can be attributed to the intrinsic state emission [76, 80, 81], electron–hole recombination [82, 83], and defective state emission [84]. Intrinsic emission of BMH is speculated to have originated from isolated sp 2 luminescence centers embedded within the sp 3 matrix of the carbonaceous film. The sp 3 matrix of graphene oxides is composed of C–OH, C–O–C, and C = O edge sites, whose energy levels lie between the energy levels of π–π* states of the sp 2 C = C domain, thus giving rise to multiple absorption bands. Both BMH01 and BMH03 possessed a strong UV absorbance band at ca. 240 nm, corresponding to the π to π* transition of C = C within the graphene oxides domain. With the increasing ratio of hexamethylenetetramine in the BMH03 sample, an additional bump at 288 nm emerged, which can be ascribed to the n-π* transition of the C = O and C = N bonds of the oxidized graphitic region [77, 85, 86]. The latter absorbance band at ca. 288 nm was induced by oxygen, nitrogen, and boron defect sites, creating new radiative recombination sites [82, 87,88,89]. Upon photoexcitation at 365 nm, BMH01 and BMH03 exhibited a maximum emission wavelengths at 429 and 447 nm, respectively. The BMH03 sample showed a slight red-shift emission, unlike BMH01 due to the greater extent of graphitization [77, 84] (Additional file 1:Figs. 5 and 6). Both BMH samples revealed a broad and much lower energy emission wavelengths than BGH samples due to lower energy emissive centers arising from O, N, and B defects and surface states. According to the electron–hole recombination mechanism, these photoexcited electrons from each defect state recombine with their corresponding holes in the HOMO, thus yielding a broad photoluminescence emission [90] (Fig. 6b). Interestingly, only BMH01 exhibited a pronounced excitation-dependent photoluminescence as observed in other BCNO [72] and carbon quantum dot systems [76]. The presence of O, and N impurities embedded within the graphene oxides matrix was shown to create a large number of surface emissive traps that corresponded to a diverse energy levels within the bandgap, thus yielding an excitation dependent fluorescence spectra in BMH01 (Additional file 1:Fig. S15). In contrast, the lack of an excitation dependent emission in BMH03 could be explained by the formation of a greater extent of graphitization (C = C) with a concurrent reduction in the surface states population (eg.:C = O) [44].
The bandgap values of BMH and BGH prepared in this study were estimated from Tauc's formulation:(αhν) 2 − hν, where α is the absorbance (Fig. 6d). The bandgap was estimated by extrapolating the photon energy intercept at (αhν) 2 = 0. For the BMH series, the presence of multiple energy levels within the optical bandgap may have originated from the electronic transition from various π-π* (C = C bonds) and n-π* of C = O or other surface groups [76, 82, 87]. As for the BGH series, carbon substituted on boron sites (CB), nitrogen-vacancy sites (VN), and interstitial carbon defect levels gave rise to the emergence of interband states between the bandgap of hBN. The presence of multiple energy levels was supported by the photoluminescence spectra of lower energy radiative recombinations [24, 47, 66] (Fig. 6b and Table 2). The BGH03 sample exhibited two large bandgaps at 5.7 eV and 3.8 eV, corresponding to the bandgap of hBN and the transition from the valence band to VN levels, respectively [50, 74] (Additional file 1:Fig. S14).
Long-lived charge carriers that can persist into the microseconds and milliseconds timescales in semiconductor photoelectrodes such as CN photocatalysts, have been proposed as an important parameter in enhancing photocatalytic activity by reducing charge recombinations [91,92,93,94,95]. Time-resolved photoluminescence (TRPL) experiments were conducted to gain insight into the recombination processes of the photogenerated charge carriers of BCNO (Fig. 6c). The µs-PL decay kinetics could be fitted with three exponential decays according to the following equation.
$$I\left( t \right) =I_{1} \exp ( - t/\tau_{1} ) + I_{2} \exp ( - t/\tau_{2} ) + I_{3} \exp \left( {t/\tau_{3} } \right)$$The multiple exponential decays imply that BCNO undergoes complex recombination from both intrinsic and defect states of BCNO [32, 72]. In the equation, I 1 through I 3 are constants with values of emission intensity measured at t =0, andτ 1 through τ 3 are the lifetimes of three channels responsible for the decay, respectively. Through multiexponential fitting of the entire decay curves for the BMH and BGH series, the average lifetimes were calculated to be 1.58, 2.10, 5.18, and 8.14 µs for BGH01, BGH03, BMH01, and BMH03, respectively. The values of I and τ in Eq. 1 for the BMH and BGH series are reported in Additional file 1:Table S5. The persistent lifetime of the charge carrier in the BCNO system has been attributed to the presence of shallow traps composed of nitrogen-vacancy (VN) stabilized by carbon impurities, which were located ca. 0.7 eV-1.0 eV below the conduction band of h-BN [72, 74]. Shallow traps in CN photocatalysts have been attributed to charge separation states with long life-times due to chemical defects [95]. According to works related to prolonged photoluminescence in CN and other nanostructured photoelectrodes [91, 92], the microseconds lifetimes of BCNO-doped graphene oxides and BCNO-doped hBN are associated with the enhanced charged separation within the BCNO domain. The ultralong lifetimes are speculated to be a critical factor in facilitating heterogeneous photocatalysis [91].
Photocatalytic Dye Degradation
As a proof of concept demonstration, the photocatalytic performance of BMH and BGH was evaluated by the photodegradation of methylene blue (MB) under UV–visible light irradiation. Details of the experimental procedure and analysis of the photocatalytic dye degradation are available in the ESI. Figure 7 shows the percent degradation (C /C 0 × 100%) for BMH03 (red line), BMH01 (blue line), BGH01 (green line), BGH03 (orange line), and CN (purple line), where C is the concentration of MB at a time, t and C 0 is the initial concentration MB after the dark equilibrium. According to the Langmuir–Hinshelwood model, ln(C /C 0) = kt , where k is the rate constant, the dye degradation rate constant values were calculated to be 2.31 × 10 −3 min −1 for BMH03, 1.52 × 10 −3 min −1 for BGH01, 1.48 × 10 −3 min −1 for BMH01 and 9.38 × 10 −4 min −1 for BGH03. Compared to the state-of-the-art metal-free photocatalyst, BMH03 exhibited a 25% improvement in the photocatalytic dye degradation rates (Fig. 7). This proof-of-concept demonstration warrants a more in-depth investigation of the structure–property relationship of this new metal-free, boron-based photocatalyst.
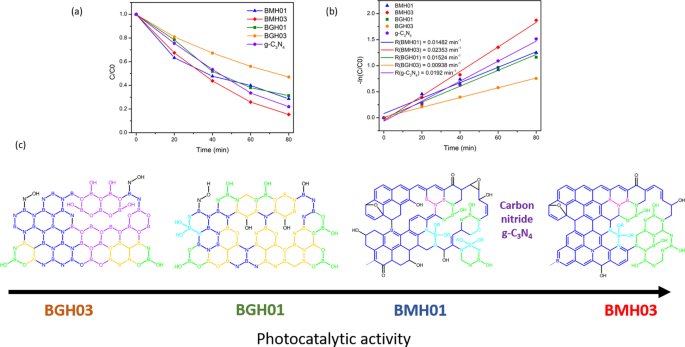
UV–visible light-induced photocatalytic MB degradation using BGH and BMH. a Photodegradation of MB under UV–visible light (plot of C /C 0) and b pseudo-first-order rate reaction kinetics for MB dye using BGH, BMH, and CN as a photocatalyst. c The proposed structures for BGH01, BGH03, BMH01, and BMH03 and their corresponding activities toward photodegradation of MB dye. Each domain is color-coded, i.e., BCNO (orange), h-BN and graphene oxides (blue), tricoordinate boron BN2(OH) and BO2(OH) (green), boroxol ring (purple), tetracoordinate B sites (aqua blue), and functional groups or dangling bonds (black).
Based on the detailed structural analysis and the proposed structure in Fig. 7c, the highest photocatalytic activity of BMH03 consisted of BCNO-doped graphene oxides [1, 65]. This ternary metal-free photocatalyst is reported to enhance the photocatalytic performances by increasing the charge separation and migration to the reaction site [1]. Additionally, the incorporation of boron into graphene-based materials [11, 20], CN [21, 96], and carbon nanotubes [97] has also exhibited enhanced performance compared to their pristine material without a dopant due to multiple synergistic effects. The large differences in electronegativity between boron, carbon, and nitrogen (2.04 vs. 2.55 vs. 3.04, respectively) yielded a strongly polarized bonding towards C and N atoms. As a result, a local positive charge was formed on boron that turned boron into a strong acidic defect site for preferential adsorption sites of pollutants [98], O2 [97], HOO
−
and OH
−
[99, 100]. Therefore, electron-rich pollutants such as MB (used as a model reaction) were speculated to preferentially adsorbed onto the electropositive B sites of the BMH photocatalyst. The photocatalytic degradation of MB on BCNO was proposed to undergo an indirect dye degradation mechanism [101]. In the indirect photodegradation mechanism, the photogenerated holes on the surface of BCNO produced highly oxidative hydroxyl radicals and attack the 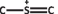 bond of MB. Meanwhile, photogenerated electrons from the conduction band of BCNO formed highly reducing superoxide radical anion O2
−
species that could directly attack MB. Figure 8 illustrates the proposed mechanism of BCNO in photocatalyzing the degradation of MB
bond of MB. Meanwhile, photogenerated electrons from the conduction band of BCNO formed highly reducing superoxide radical anion O2
−
species that could directly attack MB. Figure 8 illustrates the proposed mechanism of BCNO in photocatalyzing the degradation of MB
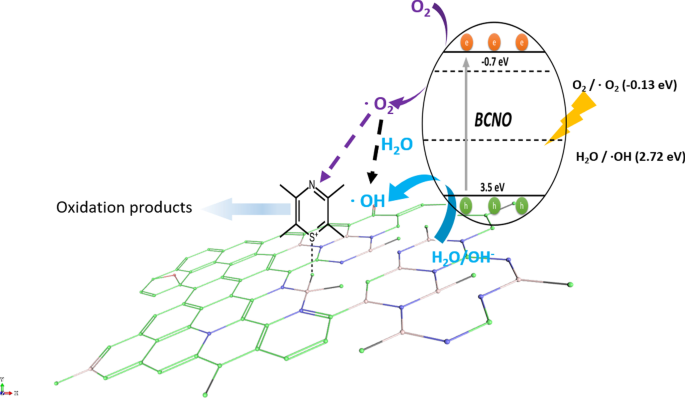
Schematic illustration of the proposed mechanism of BCNO in catalyzing the degradation of MB upon light irradiation. The positively charged MB was selectively absorbed on the N2B-OH sites. The band structures of BMH03 were determined via UPS and Tauc plot (Fig. 6 and Additional file 1:Fig. S17). The redox potentials of O2/O2 = − 0.13 eV vs ENHE and H2O/OH = 2.72 eV vs. ENHE are given in black dashed lines as reference
According to the XPS and solid-state NMR analyses, BMH03 possessed a higher composition of graphitic sp 2 C = C with a simultaneous reduction in tetracoordinate B site compared to BMH01 (Additional file 1:Fig. S9 and Table S4) The high composition of tetracoordinate B-sites in BMH01 (33%) also translated to a reduction in the number of boron Lewis acid sites serving as the active catalytic center, which explains the trend of photocatalytic activities among the BMH series [13]. However, the BGH series comprised the C, and O-doped h-BN domain with various tri- and tetracoordinate boron sites. Interestingly, BGH01 annealed at a lower temperature (Table 1, BGH01LT) was found to be inactive but exhibited a high absorbing ability in removing dye from the solution (Additional file 1:Fig. S1 for TEM morphology; Fig. S16 for photodegradation MB UV–vis absorbance).
The previous report also concluded that BCNO was photocatalytically inactive towards dye degradation but exhibited an excellent absorbing ability in removing dye from the solution [102]. The inactive BGH01LT was comprised primarily of tetracoordinate B sites and boron oxides-related bonding. Based on the SOLA analysis and the literature , the tricoordinate boron site with a δ iso of 16.7 ppm was related to boroxol rings (B2O3) [54, 56]. Our results are also supported by other works on the catalytic activation of peroxymonosulfate using amorphous boron [22]. However, the photocatalytic active BGH01, BMH01, and BMH03 possessed a high composition of BN(OH)2 and BN2OH bonding sites. Our investigation suggested that the photocatalytic activity of BCNO is highly dependent on the local structure of the boron site. While the exact catalytic site and mechanism are yet to be explored, we proposed that BNx (OH)3−x served as one of the BGH and BMH series catalytic sites. For the BMH series, the formation of tetracoordinate B-O sites was detrimental in catalyzing dye degradation due to the reduction in the Lewis acid site. At the same time, the increasing composition of the sp 2 C = C graphitic domain enhanced the photocatalytic activities of the BGH and BMH series.
Conclusions
In summary, BCNO structures and their photocatalytic activities have been presented here and found to be highly dependent on the choice of precursor, precursor ratios, annealing temperatures, and times. In this study, two types of distinctly different BCNO nanostructures were prepared via low-temperature annealing (600 °C–800 °C):(1) BCNO-doped boron nitride and (2) BCNO-doped graphene oxides. Through systematic investigation of using two different nitrogen precursors, crystalline BCNO with a quasi-spherical shape was prepared at 800 °C for 12 hr using guanidine hydrochloride as the nitrogen source. This series of BCNO exhibited moderate photocatalytic activity through the emergence of BN2(OH) or BN(OH)2 tricoordinate boron serving as the Lewis acidic site. However, BCNO prepared using melamine as the nitrogen source at 600 °C yielded multi-layered sheets with ill-defined shapes. These BCNO-doped graphene oxides layered structures exhibited the highest photocatalytic activity, surpassing the state-of-art metal-free photocatalyst, CN. For the melamine-derived BCNO layered structures, the presence of tricoordinate boron species as BN2(OH) or BN(OH)2 and a higher composition of graphitic sp 2 C = C were speculated to play an important role in promoting their photocatalytic activity. This study demonstrates the potential of BCNO as a photocatalyst for energy conversion and environmental remediation applications. Further structural optimization on this new B-C-N–O photocatalyst system is expected to facilitate the development of a sustainable catalyst for applications including solar hydrogen fuel production, environmental remediation, electrocatalytic oxygen reduction reaction, and catalytic oxidative dehydrogenation reaction.
Availability of Data and Materials
All data are fully available without restriction.
Abbreviations
- BCNO:
-
Boron carbon oxynitride
- CN:
-
Carbon nitride
- hBN:
-
Hexagonal boron nitride
- TRPL:
-
Time-resolved photoluminescence
- MAS:
-
Magic angle spin
- CP:
-
Cross polarization
- SOLA:
-
Solid line shape analysis
- I :
-
Intensity
- ATR:
-
Attenuated total reflectance
纳米材料


