废水和水处理技术
废水和水处理技术
几个世纪以来,廉价而丰富的水一直是钢铁工业认为理所当然的生产工具。但在目前的情况下,由于淡水供应和消耗之间的不平衡日益严重,水资源变得越来越稀缺,因此获得清洁和安全的水已成为现代社会的主要挑战之一。由于 (i) 人口增加和迁移到干旱易发地区,(ii) 工业快速发展和人均用水量增加,以及 (iii) 气候变化导致人口稠密地区的天气模式发生变化,水需求持续增加。这使钢铁行业进入了一个新的节水时代。此外,在过去的三十年里,世界各地对环境污染的担忧日益增加,这导致颁布了更具限制性的环境法规。
钢铁工业使用大量淡水用于各种用途,包括冷却、抑尘、清洁、温度控制(热处理)、废料(灰烬、污泥和水垢等)的运输以及其他用途。水是一些钢铁厂过程的重要组成部分,例如添加水以控制炼焦煤混合物的水分含量、烧结混合物的球团化、铁矿石球团生产过程中生球团的制造、蒸汽的生产和电力的生产,高炉渣造粒等。大量水的使用也会产生大量废水,需外排。钢铁工业废水的排放已被公认为水体污染的因素之一。钢铁厂生产过程中产生的废水中含有许多溶解和不溶解的物质和化学物质。
钢铁厂工艺对工艺用水的主要用途包括工艺尾气的冷却和净化、焦炭和炉渣的直接冷却、钢的直接冷却和净化、产品的冲洗、工艺溶液的补充和工艺设备的直接冷却等。 . 钢铁厂使用的水大部分用于加工设备的非接触冷却。水还用于蒸汽和发电。
工艺废水被定义为与钢铁生产的工艺、产品、副产品或原材料直接接触的任何废水。工艺废水还包括来自炉渣淬火、设备清洗、空气污染控制装置、冲洗水和受污染的冷却水的废水。生活废水和雨水不被视为工艺废水。非接触冷却废水是指不直接接触工艺、产品、副产品或原材料的冷却水。该废水不被视为工艺废水。非工艺废水是由非工艺操作产生的废水,例如公用事业废水(水处理残留物、锅炉排污、热回收设备产生的空气污染控制废水和热电联产设施产生的水)、处理或未处理的废水地下水修复系统、建筑地基脱水以及其他与生产过程无关的废水流。
废水排放是指将已处理或未处理的废水排放到接收流中。排放可能来自处理厂或收集系统中的溢流。未经处理的废水排放会产生多种不良情况。这些包括 (i) 河流中的氧气消耗和气味产生,(ii) 病原微生物的存在对人类健康造成的负面影响,(iii) 淤泥和浮渣堆积,(iv) 由于水体生长导致水体富营养化水生植物和藻类,因为废水中可能含有一定量的营养物质,以及 (v) 由于废水中存在的有机化合物的分解,会产生大量恶臭气体。如果废水在排放前没有得到适当的处理和无害化,这种废水的排放会加剧该地区水体的污染。因此,废水在离开厂区并排放到天然水体之前必须进行处理。
废水处理方法最初是针对废水排放到环境中造成的不利条件和对公众健康的关注而开发的。钢铁行业的废水处理相当复杂,因为废水的性质因钢铁厂的各个处理单元而异。
纯水由 2 份氢气和 1 份氧气组成。在自然界中,水含有许多溶解的杂质。事实上,水被称为“万能溶剂”,因为它能够溶解许多物质。即使是蒸馏水和雨水也不是“完全”纯净的,因为它们通常含有非常低水平的溶解物质,如氨,这些物质被认为是杂质。在地表水和地下水中发现了溶解物质。随着降雨,氮气和其他气体被吸收。水在穿过地面时,可以溶解来自地球的物质,例如钠、钙、铁、磷、镁和硫酸盐。
未经处理的新鲜生活水或生水有霉味,pH 值范围为 6.5 至 8,呈灰褐色。通常在未经处理的水中发现的污染物可以大致分为四个基本类别,即(i)有机污染物,(ii)无机污染物,(iii)病原体和(iv)其他污染物。废水中存在的典型污染物如下所示。
固体 – 废水中的总固体可以是溶解固体或悬浮固体。悬浮固体可以是胶体固体(不能沉降)或可沉降固体的形式。当未经处理的废水排放到水生环境中时,悬浮固体 (SS) 会导致污泥沉积和厌氧条件的发展。总悬浮固体 (TSS) 包括通过过滤器的所有颗粒。随着 TSS 水平的增加,水体开始失去支持多种水生生物的能力。悬浮固体从阳光中吸收热量,从而提高水温并随后降低溶解氧的水平。一些固体也可以是可漂浮的固体。这些可漂浮的固体通常由油或油脂颗粒组成并构成浮渣。浮渣最容易被表面撇渣设备去除。
可生物降解的有机物 – 可生物降解的有机物主要由蛋白质、碳水化合物和脂肪组成。可生物降解的有机物主要根据 BOD(生化需氧量)和 COD(化学需氧量)来衡量。 BOD 是衡量水质的一项重要指标,可测量细菌和其他生物体在 20 摄氏度的温度下,在 5 天的时间内氧化水样中存在的有机物所需的氧气量。 COD 测量除在反应中未完全氧化的一些芳烃(苯、甲苯和苯酚等)之外的所有有机碳。 COD是一种化学氧化反应。高 BOD 和 COD 导致水体中的氧气浓度低,并共同对水体的水生生物产生不利影响。生物可降解有机物的生物稳定性会导致天然氧气资源的枯竭,如果未经处理就排放到环境中,会导致化粪池条件的发展。
病原体 – 病原体是引起或可能引起疾病的微生物。废水中的病原微生物可以传播传染病。
营养素 – 氮和磷以及碳都是生长所必需的营养素。当排放到水生环境中时,这些营养物质会导致不良水生生物的生长。当它们在陆地上过量排放时,也会导致地下水污染。
关键污染物 – 这些是有机和无机化合物,具有引起未知或可疑致癌性、致突变性或高急性毒性的特征。出于公共卫生原因和保护生物处理过程,应尽量减少废水中这些化合物的存在。
耐火有机物 – 这些有机物往往会抵抗传统的废水处理方法。典型的例子包括表面活性剂、酚类和农业杀虫剂。其中一些可能对生物处理过程有毒。
重金属 – 重金属可能存在于钢铁厂各个单元产生的废水中。如果废水排放到用作饮用水源的水体中,则需要去除这些重金属。重金属的存在也会影响农田生物固体(稳定化废污泥)的循环利用。
溶解的无机物 – 某些钢铁厂的废水中可能存在钙、钠和硫酸盐等无机成分。如果废水排放到用作饮用水源的水体中,这些将被去除。
溶解化学品 – 根据工艺的不同,废水中可能存在大量溶解的化学物质。这需要仔细评估污染物的类型、浓度、流动性和生物降解的难易程度。排放的水中每升浓度低至 1 毫克就会产生有色水,影响水体的美学质量和透明度。它还影响光合作用。酸和碱会产生低或高 pH 值的情况。一些化学品很难通过常规处理工艺降解。
源自化合物的有机污染物含有碳。这些污染物可以生物降解,这意味着污染物可以被细菌和其他微生物消耗。在被消耗的过程中,这些有机物会产生需氧量,可以用废水的 BOD 来衡量。一些有机污染物(难降解有机物)对生物降解具有抵抗力。无机污染物不可生物降解,但可以是微生物生存所必需的营养物质。这些通常是化合物(关键污染物)或金属,它们以悬浮固体或溶解无机物的形式存在于废水中。
病原体是引起疾病的生物体,包括细菌和病毒,它们可以通过人类或动物的排泄物或处理不当的医院排泄物沉积在废水中。在废水周围工作时,适当的卫生非常重要。其他杂质可能是热废物。带有热废物的废水排放会导致进水流量和温度突然升高。热废物的典型来源是非接触式冷却水(温度超过水流温度的热水)。根据水流的用途,可以对废水的温度进行限制,以防止水流温度升高和影响使用。放射性废物可能来自使用放射源的实验室和仪器。不允许将放射性废物排放到下水道系统中通常是一种良好的做法。
各种水处理过程主要具有三个目标,即 (i) 赋予和保持取水源水的固有物理、化学和生物质量,使其适合特定用途,例如饮用水和生产过程中使用的水(ii) 允许进行废水处理,保护公众免受健康风险,而不会对环境造成任何损害,以及 (iii) 在自然环境中赋予和保存水的特性,这些特性对于水生生物的保护和发展是必要的生命和植被,以及为牛和野生动物提供饮用水,或用于娱乐和审美目的。
水或废水的处理依赖于许多单独的单元操作,这些操作组合起来形成一个过程,通常称为过程处理方案。单位的运作都基于相对狭窄的管理原则。相同的基础机制适用于该过程,无论是要净化以供饮用的来自地面、湖泊、水库、河流或海洋的水,还是要净化的废水(即污水或工业废水)安全排放到环境中。
传统的废水处理技术提高了排放到环境中的废水的质量,并抑制了污染水对其他可用清洁水资源的污染。然而,这些处理技术并不能使废水在更靠近产生点的社区中用于进一步的有益用途。需要能够进一步提高废水质量的创新和先进技术来克服传统技术的这一局限性,并促进回收和再利用实践的广泛采用。
高级处理过程可以是生物过程、物理化学过程或两者的组合(混合过程)。去除氮和磷等营养污染物的生物过程为进一步处理废水以达到可重复使用的质量提供了平台。物理化学过程,如深床过滤、浮动介质过滤和膜过滤,在中水回用处理技术中发挥着重要作用。与其他工艺相比,膜过滤具有显着优势,因为它们产生高质量的流出物,几乎不需要或不需要消毒,并且产生的污泥最少。混合过程试图一步获得生物和物理化学过程的好处。
由于废水的回收和引入净化和提纯水的工艺通常补充了保护环境的最初目标,因此各种工艺被认为属于同一领域。水处理技术可分为四个一般领域,即(i)物理方法,(ii)化学方法,(iii)生物方法和(iv)能源密集型方法。
物理过程在废水流过筛网或过滤介质时从废水中去除固体,或者通过重力沉降或气浮去除固体。被空气夹带的颗粒漂浮到表面并且可以被去除。废水处理的物理方法代表了可在很大程度上被称为固液分离技术的一系列技术,其中过滤起主要作用。过滤技术可分为常规和非常规两大类。该技术是饮用水和废水处理应用的一个组成部分。然而,它只是现代水处理厂方案中的一个单元过程,根据处理的最终目标,有多种设备和技术可供选择。要了解过滤的作用,重要的是不仅要区分与废水清洁和净化中采用的其他技术,还要区分不同单元工艺的目标。
在废水处理中使用化学品来改变污染物,从而提高去除它们的能力。改变可以包括形成絮状物或更重的颗粒质量,以改善物理过程的去除。化学处理方法依赖于需要从水中去除的污染物的化学相互作用。化学品的应用要么有助于从水中分离污染物,要么有助于破坏或中和与污染物相关的有害影响。化学处理方法既可以作为独立技术应用,也可以作为物理方法处理过程的一个组成部分。通常化学添加和物理过程一起使用来提供处理。
生物处理过程是使用微生物降解废水中的有机污染物的系统。在废水处理中,自然生物降解过程已被包含并加速在系统中以去除有机物质和营养物。微生物代谢营养物质、胶体和溶解的有机物,产生经过处理的废水。通过物理过程从处理过的废水中去除多余的微生物生长。生物工艺是首选的处理方式,因为它们在能源消耗和化学品使用方面具有成本效益。
在能源密集型技术中,热法在水处理应用中具有双重作用。它们可以用作杀菌手段,从而提供高质量的饮用水,和/或这些技术可以应用于水处理应用产生的固体废物或污泥的处理。在后一种情况下,热法可以以与它们应用于调节水的方式基本相同的方式应用,即对被有机污染物污染的污泥进行消毒,和/或这些技术可以应用于减容。减少体积是一个关键步骤,因为最终要在污染水和危险固体废物之间进行权衡。能源密集型技术包括电化学技术,这些技术大体上应用于饮用水应用。它们代表了水的杀菌和调理,以达到可口的质量。
所有这四个技术组都可以在水处理中组合使用,或者根据水处理的目标,它们可以选择组合使用。在每个通用技术类别中,都有一系列硬件和单独的技术可供选择。不仅从每个技术组中选择合适的单元工艺和硬件,而且从这四个组中选择硬件和单元工艺的最佳组合取决于以下因素:(i)工厂最终出水的清洁度要求, (ii) 待处理的流出水的数量和质量, (iii) 需要去除或使流出水中中性的污染物的物理和化学性质, (iv) 物理、化学和热力学处理水产生的固体废物的性质,以及(v)处理水的成本,包括固体废物的处理、加工和倾倒的成本。
钢铁工业废水的处理需要多种策略来去除不同类型的污染物。这些策略包括 (i) 去除固体,(ii) 去除油脂,(iii) 去除可生物降解的有机物,(iv) 活性污泥法,(v) 滴滤法,(vi) 有毒物质的处理, (vii) 酸和碱的处理,以及 (viii) 其他有机物的处理。钢铁厂的废水处理装置也称为污水处理厂(ETP)。
ETP 的目标是 (i) 确保向自然环境排放良好的水质,(ii) 以最低的成本最有效地去除污染物,(iii) 避免和/或减少其他环境影响,例如气味产生、气体排放、噪音产生和固体处置,(iv) 生产处理过的水以供再利用和循环利用,以及 (v) 在经济可行的情况下回收盐分。在规划 ETP 时,要考虑的要求是 (i) 所需的流出污水质量或符合国家、州、地方和/或组织指南的许可要求,(ii) 需要处理的污水量,(iii) 生产能力车间,(iv) 技术的复杂性、操作的便利性、适应性、可靠性和稳健性,以及能源需求,(v) 资本和运营成本,(vi) 可用土地面积,以及 (vii) 污泥产生和处置需求量.
钢铁厂各个车间产生的废水的处理通常在生产区专门建造的污水处理厂中进行。在此类 ETP 中进行的废水处理赋予了废水这样的特性,使其可以安全地从工厂排放到水体中,或者可以完全或部分循环回工艺过程。
来自钢铁厂不同工艺的废水根据其特性进行不同的处理选择。物理、化学、生物和能源密集型技术的不同处理工艺组合用于去除废水中的固体、有机物,有时还包括营养物质。
钢铁厂的废水处理工艺(图 1)与前面描述的一样分为四组。处理分为初级、初级、二级和三级四个层次。这些级别描述了废水处理的不同程度。下面简要介绍这些过程。
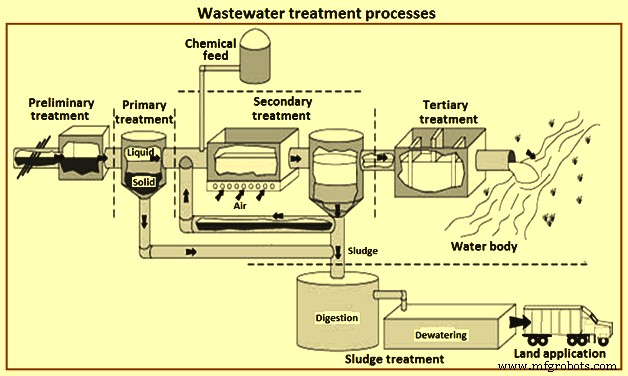
图1废水处理流程
初步处理
预处理的目的是去除可能导致堵塞、下游设备堵塞和设备磨损的材料。初步处理通常在废水送入 ETP 之前进行,以提高 ETP 的性能。在该处理中,去除废水中的粗固体和其他大物质。去除这些材料对于提高后续处理单元的操作和维护效率至关重要。在这种废水处理中,使用了许多单元过程来消除废水的不良特性。这些通常包括 (i) 气味控制,和 (ii) 诸如预曝气、粗固体研磨和使用筛网和格栅等去除大块材料等操作。很多时候,去除油和油脂以及 pH 值也进行了修正。
初级治疗
它是废水处理过程的第一步或预处理后的第二步。初级处理在初步处理之后,包括物理和化学处理,以将 pH 从碱性条件校正到接近中和的 pH 值,以及在初级澄清池中物理沉降悬浮固体,以减少下游工艺的 BOD 和 SS 负荷。总体而言,采用初级澄清装置对下游生物工艺操作的问题较少。例如,生物反应器中的油脂和生物质积累量较少,从而最大限度地减少罐中可能的沉降,并减少活性污泥生物质“非丝状”膨胀的趋势等。在大多数情况下,初级处理使用混凝——用于改善固体分离的絮凝过程。
总体而言,采用一级澄清装置对下游生物工艺操作的问题较少。初级处理的目的是通过沉淀去除可沉降的有机和无机固体,通过撇去去除漂浮物。在初级处理期间,大约 35% 至 55% 的总内向 BOD、大约 55% 至 75% 的总 SS 以及大约 70% 的油和油脂通常被去除。初沉过程中除去了少量的有机磷和有机氮以及与固体相关的重金属,但不影响胶体和溶解成分。
在初级处理期间,使用初级澄清器从废水中物理分离悬浮固体。在此处理过程中,TSS 和相关的 BOD 水平降低,废物为废水处理的下一步做好准备。通过物料的沉降和撇去去除可沉降的有机和无机固体是该处理步骤的主要目标。
初级处理涉及各种物理化学过程,并确保后续处理过程的性能令人满意。初级处理的主要工艺为沉降,辅助工艺为细筛和絮凝浮选。絮凝前通常用石灰、明矾或专有化学品进行化学处理。这种处理的主要目的是通过沉淀去除金属,同时去除一些相关的胶体BOD以产生化学污泥。初级处理采用混凝-絮凝工艺来改善固体分离。其中一些过程如下所述。
絮凝 – 这是一种物理化学过程,通过物理混合和辅助化学凝结剂,有助于促进粘性胶体和精细分离的悬浮物质的聚集。该工艺由快速混合罐和絮凝罐组成。废水流在快速混合罐中与凝结剂混合,然后通过絮凝池,在絮凝池中缓慢混合废物,使颗粒以更可沉降和更重的固体形式收集。在扩散空气或机械桨的帮助下,可以促进更好的混合。天然有机聚合物、无机电解质和合成聚电解质是用于凝结的各种不同类型的化学品。根据污染物的特性和化学性质,选择特定的化学品。
沉淀 – 初级沉淀的主要目的是分离废水中的固相和液相部分。它使用重力去除容易沉降的固体。固体主要是有机物以及漂浮的物质,如脂肪、油和油脂。沉降的固体被称为初沉污泥。因此,该工艺降低了流入废水的 SS 含量。尽管初沉污泥的体积仅占总进水废水体积的 2% 左右,但它构成了约 30% 至 40% 的有机负荷(以 COD 表示)和约 40% 至 60% 的 SS 负荷。用于去除油脂和漂浮固体的挡板和撇油器包含在沉淀室中,也可以有机械刮刀从室底部去除污泥。
固体去除效率取决于沉淀池或澄清池的特性。沉淀池是一种设备,包括用于耗散能量的入口挡板、用于颗粒沉降的静止区和用于去除沉降固体的机械装置,以及出口的低流速。
絮凝沉淀池可以是矩形、圆形或斜板(Lamella),根据当地现场条件、可用面积和设计团队的经验选择。理想情况下,需要两个或更多罐。与圆形水箱相比,矩形水箱和薄层水箱占用的土地面积更少,并且在可用土地较少的情况下非常有用。
矩形罐具有直流模式,以增加絮凝(在化学辅助沉降中)并减少停留时间。水从一端进入,通过入口挡板装置并穿过水箱的长度到达流出堰和槽。它们被设计成具有 3:1 到 5:1 的长宽比,提供了一个与理想条件非常相似的大的有效沉降区,以及 1% 的底部坡度。底部的机械刮板移动将沉降的污泥收集到收集区。污泥随后被泵出。
在圆形澄清池(图 2)中,流动模式是径向的。为了实现径向流动模式,在大多数设计中,废水被引入罐的中心或有时在罐的外围。在中心设计中,废水通过被称为“中心井”的管道和中心挡板输送,并径向流向环绕水箱周边的堰。中心井的直径通常在罐总直径的 15% 至 25% 之间,高度为 1 m 至 2.5 m。静沉区应足够大,以满足离散和絮凝沉降的溢流率和深度要求。
处理后的水通过 V 型缺口堰板排出。地板是倾斜的,以帮助污泥浓缩和清除。使用机械耙去除污泥。在沉淀池中的典型停留时间为 2 小时至 3 小时。悬浮物去除率为 45 % 至 55 %。
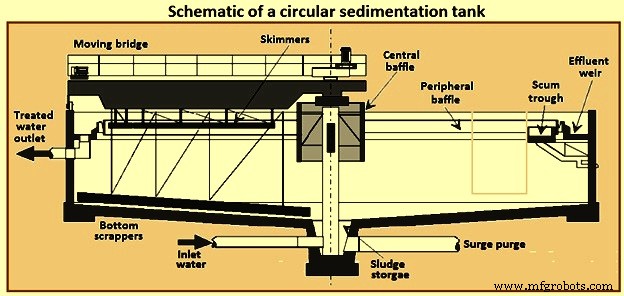
图2圆形沉淀池示意图
溶气浮选 – 在此过程中使用气泡。需要它们将废水中的悬浮颗粒提高到表面水平,以便可以轻松收集和去除悬浮颗粒。引入废水中的气泡主要附着在颗粒上,帮助其漂浮。含油废水和其他一些废水中的悬浮物、分散油和油脂可以通过溶气气浮(DAF)工艺去除。
对于油和油脂的去除,溶气气浮非常适合,尤其是在悬浮固体的比重接近 1.0 的情况下。 DAF 工艺使用加压空气释放附着在颗粒上的微小气泡(直径为 10 微米至 50 微米),使游离油颗粒很容易上升到表面然后被撇去。 DAF 工艺在去除油和油脂方面非常有效,因为油不会自然沉降,其比重小于水的比重。当油以乳化形式存在时,它需要化学品来破坏油乳液层的稳定性。
The pressurized water flow can be the entire inflow of wastewater, part of the inlet flow, or water already treated by the process (effluent). This results in dissolved air flotation to be three types of usable process, called full flow, partial flow or recirculated flow respectively. Fig 3 shows a schematic of the DAF process. The most common DAF application for wastewater treatment is a recirculated flow system, as it needs less equipment for pressurization (lower energy consumption), it avoids pump abrasion problems, and prevents the formation of colloids and emulsions within the pumping system.
DAF process can reduce oil concentrations to 10 mg/litre to 25 mg/litre as long as the influent concentration is not greater than 500 mg/litre. DAF process operates at higher hydraulic loading rates than gravity sedimentation systems and hence detention times are shorter by 15 minutes to 30 minutes. This allows the DAF process to be more compact and has a smaller footprint. DAF process systems are available in circular or rectangular configurations.
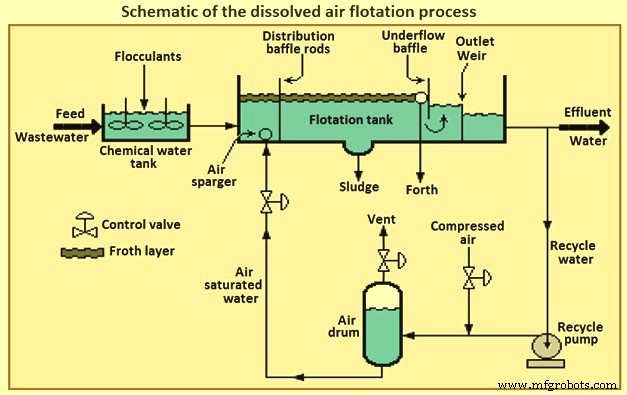
Fig 3 Schematic of the dissolved air flotation process
In retention tank the wastewater is pressurized and contacted with air. The super-saturated and pressurized water is passed through a pressure-reducing valve to the bottom of the floatation tank. The super-saturated air begins to come out in the form of fine bubbles from the solution, as and when the pressure starts releasing. The air bubbles attached with the suspended particles and trapped in sludge flock float over the surface and these floats are always swept from the surface and the mud is then collected from the bottom of the tank. The oil removal efficiency of the DAF process can be increased by the addition of certain coagulants.
Chemical treatment processes – The chemical treatment can be used, preferably before biological treatment as it removes the toxic chemicals which can kills the micro-organisms and or at any stage in the treatment process as and when it is necessary. Chemical treatment processes are described below.
Dissolved solids removal – Dissolved solids can be removed through a number of different methods namely (i) conversion to suspended materials, normally using chemicals to precipitate the contaminant as a solid or gas, to allow them to be removed by physical separation, (ii) adsorption onto a solid material, which can either be suspended or fixed as a bed, such as powdered or granular activated carbon, (iii) rejection using dense membrane processes, such as reverse osmosis or nano-filtration, or (iv) conversion to relatively innocuous end products.
Conversion necessarily involves chemistry or biochemistry, and the chemical reaction can be either reduction/oxidation (redox) or non-redox. Many chemical and biochemical processes operate by oxidation, the end products in the case of organic pollutants normally being carbon dioxide, nitrate and water. Examples of chemical reduction include the quenching of excess chlorine using bisulphite or the biochemical reduction of nitrate to nitrogen, the latter being referred to as ‘denitrification’. There also exist many important non-redox chemical processes, such as pH adjustment or precipitation of alkaline earth salts such as calcium carbonate or sulphate.
Neutralization – There is a wide range of pH of the untreated wastewater and it is not so easy to treat the wastewater with such type of varying range of the pH value. To optimize the treatment efficiency the neutralization process is used to adjust the pH value. To reduce the pH value sulphuric or hydrochloric acids can be added and to raise the pH value, dehydrated lime or sodium hydroxide alkalis can be added. Normally the process of neutralization is carried in a rapid mix holding tank or in a tank used for equalization. To control the pH of the discharge in order to meet the standards, the process of neutralization can be carried out at the end of the treatment.
Precipitation – The process of precipitation is carried out in two steps for the removal of the metal compounds from the stream of the wastewater. The mixing of precipitants with the wastewater and allowing a formation of the insoluble metal precipitants is the first step of the precipitation process. The removal of the precipitated metals from the wastewater through clarification and filtration is carried out in the second step and then the resulting sludge is being treated in a proper manner, and after treatment, it is recycled or disposed off. The important parameter to be considered in a chemical precipitation is pH controlling.
The solubility of metal hydroxides increases towards higher or lower pH and this is amphoteric in nature. Thus, for the precipitation of hydroxide for each metal, there is an optimum value of pH. As there is normally more than one metal in wastewater, hence, it is very much difficult to select the optimum treatment chemical and the pH control becomes more difficult and also it involves a transaction between the best possible removals of two or more metals. Lime, sodium hydroxide, soda ash, sodium sulphide and the ferrous sulphate are the various different chemicals used for the process of precipitation. The process for the effective removal of the metals like antimony, arsenic, chromium, copper, lead, nickel and zinc is normally the hydroxide precipitation and for removing mercury, lead, copper, silver, cadmium etc. sulphide precipitation is used. Fig 4 shows flow diagram of chemical treatment process for metal removal.
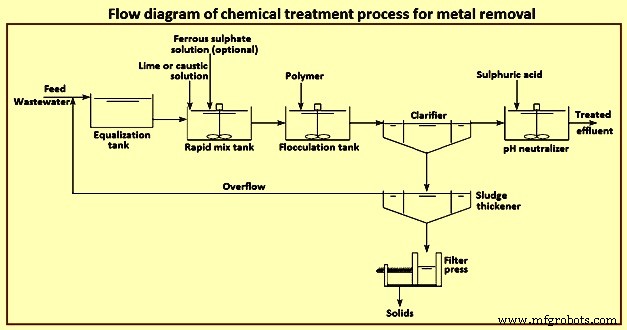
Fig 4 Flow diagram of chemical treatment process for metal removal
Secondary treatment
The secondary treatment process involves disintegration or decomposition of the suspended and dissolved organic substances present in the waste water using microorganisms. The activated sludge process (ASP) and the biological filtration methods are the mainly used biological treatment processes. The biological treatment process which is the mainly used for the secondary treatment process is based on the micro-biological action to decay the organic suspended and dissolved wastewater. The microbes can be used for the natural compound, both as a source of carbon sources and as an energy sources.
For removal of organic pollutants, the most efficient secondary treatment process is biological treatment. It primarily employs microbes naturally present in wastewater to break down organic contaminants. Some inorganic compounds like ammonia, cyanide, sulphide, sulphate and thio-cyanate are also biologically degradable. Biological processes can be broadly classified as (i) aerobic in which microbes which are used need oxygen to grow, (ii) anaerobic in which microbes which are used grow in the absence of oxygen but uses other compounds such as sulphate, phosphate or other organics present in the wastewater other than oxygen, and (iii) facultative in which microbes which are used can grow in the presence or absence of oxygen.
Aerobic processes consist of a biological reactor with a controlled amount of biomass and a clarifier for separation of the biomass from the final effluent. Aerobic processes need higher energy inputs and produce greater amounts of sludge compared to anaerobic systems. As an example, for the same 100 kg COD load entering the aerobic treatment plant, the energy needed is 100 kWh for aeration and produces 30 kg to 60 kg of sludge with the outlet effluent COD load of 2 kg to 10 kg. In the anaerobic treatment plant for the same 100 kg COD load, the sludge production is only 5 kg, or six to twelve times less, and produces 40 cum (cubic meter) to 45 cum of biogas which can be converted to produce 382 kWh of electricity. However, the outlet water COD is twice that of the aerobic plant, and hence of a lower quality.
Hydraulic retention time – It is the average time in the aeration basin equivalent to the volume of the basin divided by the average flow and expressed as hours. The hydraulic retention time is required to be sufficiently long to remove the prerequisite BOD and is dependent on the type of the biological treatment system. It can range from 0.5 hours to 120 hours. The lower the hydraulic retention time the quicker the wastewater reaches the outlet.
Mixed liquor suspended solids (MLSS) – Suspended solids level is one of the most important control parameters in biological wastewater treatment processes. It is not only directly related to sludge settling properties and effluent quality, but also related to food / micro-organism ratio which is in turn related with all aspects of sludge properties. MLSS represents the total suspended solids including bacteria, dead biomass, and higher life forms, irrespective of biological activity. The organic portion of MLSS is represented by ‘mixed liquor volatile suspended solids’ (MLVSS) which represents the biomass. MLSS is controlled by the sludge wasting rate. Typical MLSS are dependent on the process type. The more concentrated is the MLSS, the smaller is the equipment footprint and hence the popularity of membrane bioreactors (MBRs) in space constrained locations. MLVSS is 0.75 MLSS.
Food to microorganism (F/M) ratio – It is a term used for expressing the organic loading of an activated sludge process. F/M is a critical factor in process design and operation, especially in determining the aeration basin volume. F/M range is around 0.5 to 1.5. For conventional plants, F/M of 0.2 to 0.5 is aimed for. In biological treatment plants operating at high F/M loads (0.8 to 1.5), the rate of treatment increases at the cost of poor settlability of the sludge. Processes operating at low F/M loads (0.05 to 0.2) are associated with slow BOD removal rates but with good sludge settling. However, the system can be easily upset by a spike load of organics.
Sludge age – It is also known as ‘mean cell residence time’ (MCRT) and ‘solids retention time’ (SRT). It is calculated as the total quantity of sludge in the aeration tank and clarifier divided by the daily sludge losses through waste activated sludge and effluent. Sludge age can vary from 0.5 day to 75 days in low-growth rate systems. Sludge age is an indication of F/M ratios. Shorter times are indicative of high F/M ratios and longer times are indicative of low F/M ratios. Sludge age is expressed by the equation ‘sludge age =sludge mass in (aeration tank + clarifier) / daily sludge losses’.
The quality of sludge age can be determined using a microscope at 100x magnification. Daily microscopic analysis can prevent problems. Micro-organisms considered important in biological treatment are bacteria, fungi, algae, protozoa, rotifers, and worms. The presence of higher life form indicator organisms normally correlates to plant performance. They can indicate if the sludge is young, medium, or old. Good settling sludge is characterized by the presence of protozoa such as stalked ciliates and suctorians and normally is golden brown in colour (sewage treatment plants). Low sludge age is characterized by the absence of stalked ciliates and predominance of free swimming ciliates such as paramecium (these expend a lot of energy in swimming) and high BOD slugs by the absence of higher life forms. Old sludge is characterized by the presence of many worms (nematodes) or rotifers.
Another useful indicator is the ‘sludge volume index’ (SVI). Sludge is poured to a 1 litre graduated cylinder and the percentage of settled sludge in 5 minute intervals is noted for 30 minutes. SVI is expressed in ml/g. It is a reliable troubleshooting test. SVI values can vary from 30 ml/g to 400 ml/g. Values below 150 indicate good sludge settling and above this indicate sludge bulking. Other key variables which affect the operation of the biological reactor are given below.
Oxygen requirement – Oxygen is needed for the decomposition of organic matter. The concentration depends on organic matter consumption, endogenous respiration demand and total nitrification of TKN (total Kjeldahl nitrogen) oxidation. Typical oxygen concentration in an aeration tank is 2 mg/l to 4 mg/l. The higher values are maintained for nitrogen removal. Above this, electricity is wasted.
Sludge production (sludge yield) – The decay of biomass produces sludge. For conventional industrial systems, sludge production can be as low as 0.15 kg / kg BOD, such as in coke making.
Sludge recirculation rate – A portion of the sludge produced is recirculated to promote the production of more sludge in the aeration tank. It is the ratio between the sludge recirculation volumetric flow and treatment volumetric inflow. In any case, the capacity of the sludge recirculation system is not to be less than 200 % of the daily average total inflow.
Nutrient requirements (C:N:P ratio) – Besides carbon, hydrogen, and oxygen biomass needs nitrogen, phosphorous, and micro-nutrients such as iron, calcium, magnesium, copper, zinc and so on. Most industrial wastewaters lack N and P which is to be added (in the form of urea, super-phosphate or ammonium phosphate) to maintain optimal microbial growth conditions. The minimum C:N:P ratio needed for optimal microbial growth in the in aerobic processes is 100:5:1, and anaerobic processes is 330:5:1.
The most common biological processes are described briefly below.
Aerobic processes – activated sludge process – Biological processes, employing aerobic biomass in suspension, have traditionally been known as activated sludge processes. The ASP was developed in the United Kingdom in the early 1900s for the treatment of the domestic sewage and it has since been adapted for removing biodegradable organics in industrial wastewater. The ASP and its variants are capable of treating biodegradable wastewater of moderate strength (10 mg/l to 1,000 mg/l BOD) to high strength (greater than 1,000 mg/l BOD). The ASP does not remove heavy metals or TDS. Some contaminants such as cyanide, and heavy metals such as chromium and mercury, present in the wastewater act as inhibitors for the proper functioning of the ASP as well as other biological processes. ASPs have been categorized, according to the mass loading design, in three groups namely (i) low load activated sludge (extended aeration, oxidation ditches, etc.), (ii) medium load (or conventional), and (iii) high loading.
The ASP involves blending settled primary wastewater or equalized influent with a culture of micro-organisms into a fluid called ‘mixed liquor’. This mixed liquor is passed through an aeration tank which provides an adequate oxygen rich environment for the microbes to eat and stabilize the organic matter in water. Mixing brings oxygen and food to micro-organisms allowing the micro-organisms to clump together whilst preventing floc settling in the aeration tank. The process produces ‘waste activated sludge’ (WAS) consisting of microbes and excess microbial matter. The solids and treated wastewater are separated in a secondary clarifier or other solids separation step such as a membrane bioreactor (MBR). Here the majority of the WAS is returned to the aeration tank as returned activated sludge (RAS) to maintain the microbial population in the aeration tank, as well as ensuring that the activated sludge is old enough to degrade COD and aromatic hydrocarbons. The remainder is removed and undergoes thickening.
The secondary clarifier has the dual purpose of clarifying the wastewater as well as concentrating the sludge. The process is sensitive to pH fluctuations, where a high or low pH can upset the system and cause overloading of the clarifier. Fig 5 shows a schematic of the activated sludge process and membrane bioreactor process.
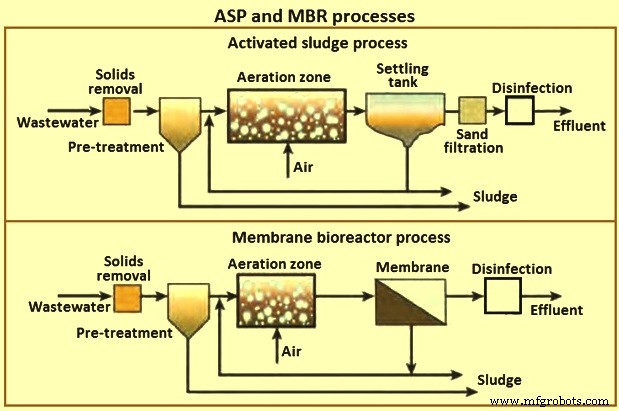
Fig 5 ASP and MBR processes
Nitrogen containing compounds are toxic to aquatic life, deplete oxygen in the receiving waters, adversely affect public health and reduce the potential for water reuse. Hence, nitrogen containing compounds are removed, if deemed excessive, by nitrification and then denitrification processes. Organic nitrogen is converted to ammonia, then converted to nitrite, which is further oxidized to nitrate and finally to gaseous nitrogen. Denitrification consumes alkalinity and needs to be sufficient so as not to depress the pH. It requires 7.14 mg/l of bicarbonate alkalinity for each 1 mg/l of ammonia nitrogen removed. Oxygen also needs to be maintained at concentrations closer to 4 mg/l for denitrification. The process control is normally customized for each effluent treatment system depending on wastewater characteristics and for optimal operation.
Aerobic processes – oxidation ditch process – This process which has been developed in the 1950s in the Netherlands, is a variant of the ASP and is a special form of extended aeration. The shape of the oxidation ditch is like a ring. Wastewater, micro-organisms and activated sludge is mixed in a continuous loop ditch in order to complete nitrification and denitrification reactions. The oxidation equipment consists of ditch body, aeration mixers and inlet and outlets. Given its long hydraulic retention time of 20 hours to 36 hours, low organic loading and long sludge age compared to conventional ASP, equalization, primary sedimentation, and sludge digestion tanks are omitted.
Oxidation ditch has many advantages in that it provides (i) low energy consumption, (ii) low maintenance, (iii) ease of operation, low capital expenditure, (iv) less sludge due to long extended solids retention time, and (v) resistance to shock loads and hydraulic surges due to long hydraulic retention time. The disadvantages are that the effluent suspended solid quality is inferior to the ASP process and needs a large land area.
Aerobic processes – sequencing batch reactor – The sequencing batch reactor (SBR) process differs from the other ASPs. It is a batch process. The principle is that all of the process steps of ASP, i.e. primary settling, biological oxidation and secondary settling take place in a single tank. The process steps are filling, react, settle, draw, and idle. SBR is compact and has low capital expenditure. It is used when land area is scarce since it needs only one tank to fulfill the aeration and clarification steps. It is also used to treat nitrogen and phosphorous. Standard cycles are normally 4 hour to 6 hours long, resulting in 4 to 6 reaction cycles per day. Compared to the conventional ASP, it is resistant to shock loading, flexible operation due to adjustment of run time and low sludge production.
Trickling filters – These filters, developed in the 1890s, are an example of a fixed film biological process compared to the ASP which is a suspended process. A trickling filter consists of bed of coarse material, such as rounded rocks (25 mm to 100 mm in diameter), crushed stone, wooden or plastic slats and plastic rings over which wastewater is discharged from moving spray distributors or fixed nozzles. The filter media provides a large amount of surface area for the micro-organisms to cling and grow a jelly like bio-film of around 10 mm thickness. In the outer portions of the bio-film (0.1 mm to 0.2 mm) the aerobic bacteria break down the organic matter. When the bio-film becomes very thick it falls off and a new bio-film layer forms. Modern trickling filters use plastic media over rocks since they weigh less and because of it, filter media can be upto 6 m in depth compared to 3 m in depth for rock filters, allowing taller filters using less land area.
The filter effluent is recycled to minimize drying of the filter media, improve filter efficiency, and reduce odour potential. Sometimes, two filters are assembled in series to handle strong wastewater. The sprays rotate at 2 revolutions per minute (rpm) to 5 rpm and a typical wetting rate is 0.6 cum/hour to 2.4 cum /hour. When the wetting rate is too low, the water does not penetrate the depth of the filter bed uniformly causing channeling and acts as an incubator for flies, as well as creating odour problems. Low rate filters operate on natural ventilation, whereas high rate filters require forced draft fans to provide adequate ventilation.
The trickling filter is followed by a secondary clarifier. Trickling filters are classified according to the organic and hydraulic loads such as low rate, intermediate, high rate, roughing filter and super high rate. The advantages of a trickling filter are (i) lower energy requirements than ASPs, (ii) simple operation with no issues of MLSS inventory control and sludge wasting, (iii) better recovery from shock toxic loads, (iv) no problems of bulking sludge in secondary clarifiers, (v) compact and suitable for place where land is scarce, (vi) less equipment maintenance needs, effective in treating high concentration of organics dependent on type of media used, and (vii) better sludge thickening properties. The disadvantages are organic loading levels, that the effluent water quality (in terms of BOD and TSS) is lower than ASP and can need further treatment, odour problems, flies, prone to plugging of filter media and at low temperatures natural ventilation systems do not operate that well.
Moving bed bioreactor – Moving bed bioreactor (MBBR) was developed in the 1980s by Kaldnes in Scandinavia. The MBBR process is a more modern fixed film process in which the micro-organisms grow on plastic media. The media are made from high density polyethylene or polypropylene with a diameter of 13 mm to 25 mm, and hence have a large surface area which helps the biomass to grow inside the surface and are in constant motion due to the compressed air which is blown from under the tank. The process has been applied in a variety of industrial wastewater treatment applications in aerobic and anaerobic modes with or without denitrification depending on the mode of mixing.
Benefits of MBBR are that it is good for high organic loading applications, improved settling characteristics, no need for sludge recirculation from secondary clarifier thereby making it a ‘once through’ process, compact and low footprint compared to the ASP process and has modular construction. It can also retrofit existing ASP systems, needs fewer operational controls than ASPs, and contains fewer mechanical and instrumentation controls compared to a MBR system. A typical hydraulic retention time for MBBR is 2 hours to 3 hours, compared to 12 hours to 24 hours for ASPs. Disadvantages of MBBRs compared to the ASP are that it needs a higher oxygen concentration, the need for improved influent wastewater screening, and additional hydraulic profile head losses due to flow through the media screening devices.
Membrane bioreactor – Though external membrane bioreactors were originally developed in the 1960s, they became popular only after the development of the immersed (submerged) MBRs in the late 1980s. The lower operating cost of the submerged MBR configuration and the decreasing cost of the membranes have made MBRs a popular choice for domestic and industrial wastewater treatment. MBRs are used for industrial wastes with BOD of 5,000 mg/l to 40,000 mg/l with BOD ranges of 200 mg/l to 600 mg/l. Fig 6 shows the principle of membrane bioreactor.
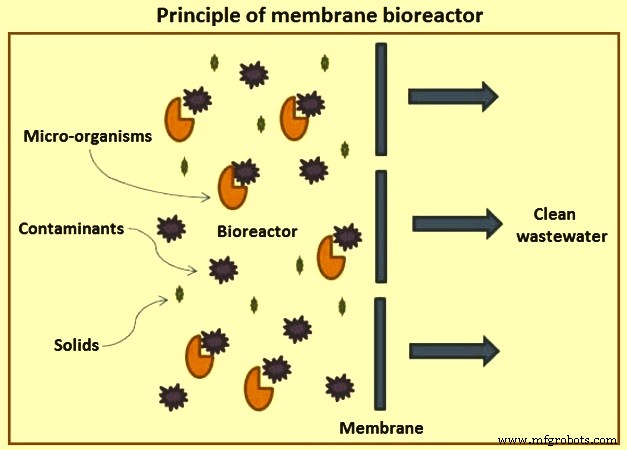
Fig 6 Principle of membrane bioreactor
The quality of the final effluent from a conventional ASP unit is highly dependent on the hydrodynamic conditions in the clarifier and settling characteristics of the sludge. This leads to variable performance. As a result, large clarifiers are needed with long residence times. The MBR process was developed to remove these disadvantages of conventional ASPs.
MBRs are a hybrid with two interdependent treatment processes:biological treatment and membrane treatment (Fig 5). It is similar to a conventional ASP in that both have mixed liquor solids in suspension in an aeration tank. The difference in the two processes lies in the method of separation of bio-solids. In the MBR process, the membranes create a solid barrier to bio-solids based on micro-filtration (MF) with a pore size of 0.6 micrometers, or ultra-filtration with a pore size of 0. 04 micrometers, and hence are not subject to gravity settling characteristics of the solids. Thus, a MBR unit brings aeration, clarification, and filtration in a single step with MLSS concentrations reaching 20,000 mg/l or higher resulting in a smaller footprint than conventional ASP units.
MBRs provide a final effluent quality independent of sludge conditions with higher removal of organics and persistent pollutants, and nutrients with COD removal of 98 % and suspended solids removal efficiency of 100 %. The high quality effluent produced is ideal for reuse applications. Another feature of MBRs is the long sludge age. However, this also contributes to fouling of membranes. Moreover, MBR units can be installed directly to a reverse osmosis (RO) plant, bypassing the need for an ion exchange or other equipment to protect a membrane plant provided the hardness or scaling compounds are not excessive.
There are two types of MBR configurations namely immersed and side-stream. Immersed systems are more common in large industrial units, whereas side-stream is limited to smaller units. There are also differences in the membrane employed from hollow fibre, flat plate, and tubular. Immersed MBRs use hollow fibre or flat plate whereas tubular membranes are used in side-stream MBRs. MBR produces an equivalent treatment level to an activated sludge process followed by micro-filtration or ultra-filtration.
Despite the advantages of MBRs, there are still challenges in using MBRs in industrial applications. The advantages of MBR are (i) 25 % lower footprint, (ii) replaces the clarifier and gravity filter of conventional systems, (iii) ideal for land constrained sites and lower hydraulic retention time of 4 hours to 8 hours. MBR provides impermeable barrier for solids producing highest quality effluent with BOD less than 5 mg/l and turbidity of less than 0.1 NTU (Nephelometric turbidity unit). Membrane fouling is one of the major challenges which results in reduced performance and frequent cleaning or membrane replacement leading to increased maintenance and operating costs. All MBRs require a minimum of fine screens of 3 mm. Sludge produced can be difficult to dewater. Sludge retention time is independent of hydraulic retention time. High sludge age of 15 days to 140 days can be obtained. It has modular expandability, less odour, and flexible operation with less susceptible to upsets. The process can be automated.
Secondary clarifiers – The purpose of the clarifier is twofold. One is to thicken the solids after biological treatment and then settle them out. The second is to produce a clear effluent of the settled solids. Clarifiers in activated sludge systems are to be designed not only for hydraulic overflow rates, but also for solids loading rates. This is because both clarification and thickening are needed in activated sludge clarifiers. Of the process variables the most important is sludge age or mean cell residence time. Another important control parameter is the solids loading rate which is defined as the required surface for suitable sludge thickening in the bottom of the unit (compression zone). The clarifiers are either of rectangular design or of circular design.
Tertiary treatment
Conventional secondary treatment frequently is not sufficient to meet the required effluent quality standards to discharge water to surface water bodies. The effluents can need tertiary processes so as to complete solids and organic matter removal, for colour reduction or recalcitrant compounds degradation, nutrient reduction, and disinfection. The persistent contaminants which the secondary treatment is not able to remove are removed by the tertiary treatment process. These processes are classified as ‘tertiary treatments’, as they are installed after secondary treatment, but some of them, like oxidation processes, can be also placed before biological treatment to improve the bio-degradability of recalcitrant compounds.
Before the treated wastewater is reused, recycled, or discharged to the environment, the tertiary treatment process is used as a final cleaning process cleaning process to improve the quality of the wastewater. For the removal of nutrient (nitrogen and phosphorus), removal of toxin [pesticides, VOC &metals], and for the polishing of the effluent like BOD &TSS, the tertiary treatment processes are used. These processes are the extension of conventional secondary biological treatment process for the further stabilization of the substances which demands oxygen in the wastewater, and also to remove the nitrogen and phosphorus.
The physical and chemical separation techniques like activated carbon adsorption, flocculation or precipitation are the process involved in the tertiary treatments. The most common tertiary treatment applications are filtration and disinfection and where applicable ammonia and phosphorous removal. Ammonia is toxic to fish and phosphorous causes algal blooms.
Filtration – Filtration is a separation process which consists in passing a solid–liquid mixture through a porous material (filter media) which retains the solids and allows the liquid filtrate to pass through. Granular media polishing filters are used the removal of suspended solids for the removal of suspended solids in the 5 mg/l to 50 mg/L range. The most common filters are the multimedia filters. The quality of the filtrate depends on the size, surface charge, and geometry of both suspended solids and filter media, as well as on the water analysis and operational parameters. Based on media filters can be categorized as (i) single media (sand or anthracite), (ii) dual media (sand and anthracite), and (iii) multimedia (garnet, sand, and anthracite).
The most common filter media in water treatment are sand and anthracite. The effective grain size for fine sand filter is in the range of 0.35 mm to 0.5 mm, and 0.7 mm to 0.8 mm for anthracite filter. In comparison to single sand filter media, dual filter media with anthracite over sand permit more penetration of the suspended matter into the filter bed, thus resulting in more efficient filtration and longer runs between cleaning. The design depth of the filter media is a minimum of 0.8 m. In the dual filter media, the filters are normally filled with 0.5 m of sand covered with 0.3 m of anthracite.
In industrial applications, filters are housed in steel pressure vessels where the interior is epoxy coated, with interior manifolds for distribution of water and an under drain system for collection of filtrate and backwashing.
As the filter vessel for pressure filtration is designed for pressurization, a higher-pressure drop can be applied for higher filter beds and / or smaller filter grains and / or higher filtration velocities. The design filtration flow rates are normally 10 m/h to 20 m/h and the backwash rates are in the range of 40 m/h to 50 m/h. The available pressure is normally about 2 bars to more than 4 bars.
For feed waters with a high fouling potential, flow rates of less than 10 m/h and / or second pass media filtration are preferred. If the flow rate has to be increased to compensate for one filter which goes out of service, the flow rate increase is to be gradual and slow to prevent the release of previously deposited particles.
During operation, influent water to be filtered enters at the top of the filter, percolates through the filter bed, and is drawn off through the collector system at the bottom. Periodically, when the differential pressure increase between the inlet and outlet of the pressure filter is 0.3 bars to 0.6 bars, the filter is backwashed and rinsed to carry away the deposited matter. Backwash time is normally about 10 minutes. Before a backwashed filter is placed back into service, it is to be rinsed to drain until the filtrate meets the specification. Backwash rates when excessive leads to loss of filter media.
Variations of the deep rate filtration are high rate filtration which operates at much faster inlet flow rates. Aside from media filters, other types of filters are disc filters and cartridge filters. These are also used to protect membrane filtration systems. Disc filters made from pleated cloth media have very high flow rates and a small footprint, producing very high quality water suitable for reuse applications and do not need extensive backwashing.
Some advanced water treatment processes are also used as the tertiary treatment. These processes are applied to the conventional treated wastewater to improve the quality upto a degree suitable for various applications of recycle and reuse including the potable reuse. The additional tertiary treatment processes are different membrane treatment processes like micro-filtration, ultra filtration, nano-filtration, other processes like reverse osmosis, advanced oxidation processes, and additional disinfection processes like ozonation and the use of ultraviolet radiation. Some of these advanced processes are described below.
Membrane technology – Membranes are a popular choice for water reuse applications since their advent in the 1960s. Costs of membrane systems have reduced dramatically and, coupled with technological advances in membrane design, membrane options and operating limits, the range of applications in water and wastewater treatment is increasing rapidly. In pressure driven membrane filtration, membranes separate the components of a fluid under pressure. The membrane pores, being extremely small, allow the selective passage of solutes. The popularity of membrane processes arises from the fact that they are effective in the removal of both dissolved and suspended solids. A wide range of materials like cellulose acetate, polyamides, poly- sulfones, poly-propylene, nylon, poly-acrylonitrile, poly-carbonate, polyvinyl alcohol, poly-tetra-fluoro-ethylene, ceramic, and metal composites are basically used to produce the membranes. The membrane pore size is the parameter for the degree of selectivity of a membrane. On the basis of the pore size, there are four types of pressure driven membranes. Micro-filtration and ultra-filtration are low pressure applications given their larger pore size. Nano-filtration needs medium pressure, and ‘reverse osmosis, given the smaller pore size, needs significant pressure to push the solute through the membrane.
Advanced oxidation processes – Advanced oxidation processes (AOPs) are defined as processes which involve generation and use of powerful but relatively non-selective hydroxyl radicals in sufficient quantities to be able to oxidize the majority of the complex chemicals present in the effluent water. The AOPs show specific advantages over conventional treatment alternatives since they can eliminate non-biodegradable organic components and avoid the need to dispose of residual sludge. After fluorine ([V (volts) =-3.06], hydroxyl free radicals (OH-) have the highest oxidation potential (V =-2.86). In the AOP process, OH – radicals are generated which in turn react with organic molecules to generate CO2 and water. AOPs can be classified into two groups, non-photochemical AOPs and photochemical AOPs. Photochemical means a light source is needed. Normally ultra violet (UV) light is used as the photo-chemical source. Low pressure UV lamps have a wavelength of 254 nm. Maximum ozone absorption takes place at a wavelength of 253.7 nm. Of the non-photo-chemical technologies, those most prevalent in the treatment are Ozonation, Ozone/ (H2O2) and Fenton’s reaction.
Ozonation – Discovered in 1785, Ozone (O3) is a widely applied strong oxidizing agent (-2.07V) for disinfection of potable water and wastewater, decolourization, odour removal, organics degradation and cyanide destruction, etc. O3 at room temperature is a bluish pungent gas, sparingly soluble in water, highly corrosive, toxic and explosive when the concentrations in air exceed 20 %. As a germicide, it is 3,125 times faster than chlorine. Ozonation efficacy is increased with high pH and temperature. When O3 dissolves in water or wastewater it can remain as the O3 molecule (at pH more than 7 and slower reaction) or decompose (pH more than 8) producing the hydroxyl free radical (OH-) which is a 35 % stronger oxidizing agent than O3. Both reactions occur simultaneously and hence reaction kinetics strongly depends on the characteristics of the treated wastewater (e.g. pH, organic concentrations, presence of foaming agents and surfactants, ozone concentration and temperature, etc.). A pH of 8 to 10 is most suitable for oxidation of organic compounds. Ozone is sparingly soluble in water and rapidly decreases with increasing temperature. At a temperature of 20 deg C, 100 % ozone solubility in water is 570 mg/l. The preferred temperature ranges from 25 deg C to 50 deg C.
A simplified reaction mechanism of ozone at a high pH is given by the equation 3 O3 + H2O =2OH radical + 4 O2. Molecular O3 is a very selective oxidant. It only reacts with certain compounds and for this reason it can be applied in low dosages for industrial wastewater applications. It can also inhibit or destroy the foaming properties of residual surfactants as well as oxidizing a good portion of the COD. Thus, O3 improves the overall biodegradability of the effluent by converting recalcitrant compounds to easily digestible compounds and can be applied upstream or downstream of a biological treatment plant. The residual oxygen in the vent gas can be recycled back to the secondary biological treatment plant, reducing aeration requirements. Another advantage is that it does not increase sludge mass.
O3 can be applied in the gaseous form and because of its unstable nature it needs to be generated on-site from air or pure oxygen using UV radiation, electrochemistry or corona discharge generators. O3 leak detectors are to be installed to give audible and visible warnings and shut down the generators in the event of a leak. O3 is deactivated in the presence of high concentration of salts. A variation of this process is the O3/H2O2 process. Developed to reduce the O3 concentrations, the H2O2 acts as a catalyst enhancing the capability of O3 to produce more OH radicals. At a low pH, H2O2 reacts very slowly with O3 and at a high pH (alkaline conditions) reacts rapidly.
制造工艺


