夹杂物、夹杂物工程和清洁钢
夹杂物、夹杂物工程和清洁钢
夹杂物是钢在生产和加工过程中形成的非金属化合物和沉淀物,因此是炼钢的副产品,由不同的化学成分和工艺产生。夹杂物的大小和组成可能有很大差异,从而产生相应的广泛影响,并要求使用复杂的分析设备进行表征。
夹杂物由嵌入钢金属基体中的玻璃陶瓷相构成。夹杂物控制是促进钢中夹杂物的去除,减少它们对钢质量和加工的有害影响。这是炼钢实践的一个重要方面。然而,某些夹杂物类型的存在也会在钢中产生有益的影响。
夹杂物的来源、去除和机械后果取决于它们的类型和工程。夹杂物的化学成分及其体积分数由生产过程中涉及的不同步骤的管理决定,例如熔化、精炼和铸造操作。因此,夹杂物的数量取决于所应用的操作参数与所生产钢种的特性之间存在的关系。
钢包中夹杂物的演变受几个因素的影响,例如出钢时炼钢炉中母体夹杂物的类型和尺寸分布、出钢时的氧气水平、炼钢炉中夹带的渣量、向钢包中添加合成渣的类型、数量和时间、添加脱氧剂的类型和时间以及钢包中搅拌的时间和强度等等。夹杂物的形状可以是球状、片状、树枝状或多面体。
球形是可取的。某些夹杂物,如 MnS(硫化锰)、固化过程中在枝晶臂之间的空间中形成的硫化氧、铝酸铁和硅酸盐是球状的。血小板形状不理想。铝脱氧钢含有沿晶界分布的薄膜形式的 MnS。多面体夹杂物不是很有害。夹杂物具有不同的形状,如下所述。球状夹杂物是最理想的,因为它们对钢的机械性能的影响适中。球状夹杂物呈球形是由于它们在液态铝含量较低时形成的。
片状夹杂物存在于被铝脱氧的钢中。这些夹杂物含有位于钢晶界的薄膜(片状)形式的 MnS 和氧硫化物。这种夹杂物是凝固过程中共晶转变的结果。片状内含物是不可取的。它们大大削弱了晶界,并对机械性能产生不利影响,特别是在热条件下(热脆性)。
枝晶状夹杂物是由于使用了过量的强脱氧剂(铝)。这导致形成树枝状氧化物和硫化物夹杂物(分离的和聚集的)。这些夹杂物的熔点高于钢的熔点。枝晶状夹杂物的锋利棱角会引起内应力的局部集中,从而大大降低钢的延展性、韧性和疲劳强度。
添加少量稀土(铈、镧)或碱土(钙、镁)元素(经铝深度脱氧后)改善枝晶状夹杂物的形态时,形成多面体夹杂物。由于其形状接近球状,多面体夹杂物对钢性能的影响小于枝晶夹杂物。
有微观夹杂物(尺寸1微米到100微米)和宏观夹杂物(尺寸大于100微米)。宏观夹杂物是有害的。微夹杂物是有益的,因为它们会限制晶粒生长,提高屈服强度和硬度。微夹杂物充当碳化物和氮化物沉淀的核。宏观夹杂物将被移除。微夹杂物可以通过将它们均匀地分散在基体中来提高强度。
夹杂物的有害影响很大程度上取决于它们的化学成分、体积分数、分散度和形态。通常,大且不易破碎的高熔点夹杂物是最不需要的夹杂物。然而,与这些夹杂物相比,更优选小而易碎的夹杂物或具有较低熔点的夹杂物。这些偏好的原因是具有较低熔点或易碎的夹杂物很可能在随后的热或冷成形过程(成形过程的影响和压缩比)或热处理中变形、压碎成更小的夹杂物或消失。钢经过铸造和凝固过程。
钢的力学行为在很大程度上受夹杂物和析出物的体积分数、尺寸、分布、成分和形态的控制,这些夹杂物和析出物充当应力提升器。夹杂物尺寸分布尤为重要,因为大的宏观夹杂物对机械性能最有害。有时灾难性缺陷是由整个钢炉中的一个大夹杂物引起的。虽然大夹杂物的数量远远超过小夹杂物,但它们的总体积分数可以更大。
清洁钢是指在尺寸、形状、成分、分布和频率方面含有有限夹杂物的钢。因此,清洁钢的性能优于其他材料,并且在高应力状态下表现出色,例如用于运输设备和其他应用的材料。
钢的清洁度是钢质量的一个重要因素,对清洁钢的需求每年都在增加。然而,冶金学家谨慎使用术语“清洁钢”。这是因为(i)不同应用对钢的清洁度要求不同,(ii)在不同操作中生产的钢的清洁度不同,以及(iii)对“清洁钢”一词的正常理解,有些人将其解释为含义钢中没有夹杂物。从操作和产品性能的角度来看,钢材的清洁度都会产生影响。
对高品质的要求不断提高,使钢铁生产企业高度重视所生产钢材的“洁净度”要求。钢铁生产商正在生产不同的钢种以满足钢铁产品的各种要求。每种要求的钢的清洁度等级取决于每个钢种的夹杂物数量、形态、成分和尺寸分布。例如,在自由加工或加硫钢中,想法不是完全去除夹杂物,而是对其进行改性以提高可加工性。因此,对每个钢种的夹杂物或清洁度的允许水平进行平衡的意见对于钢铁生产商和钢铁用户来说确实具有重要的技术和经济意义。在很大程度上,强调“清洁钢”一词是为了满足客户对非金属夹杂物特性的应用规范和要求。
随着清洁度要求的日益严格和新炼钢牌号的开发,了解夹杂物的形成和演变过程以及开发改善它们从钢水中去除的方法非常重要。在钢-夹杂物-气体体系中,夹杂物尺寸大,夹杂物与钢的界面能高,夹杂物与钢的接触角大,有利于夹杂物的去除。
洁净钢夹杂物的要求因钢种和用途而异,夹杂物工程的目标是减少有害夹杂物,促进有益夹杂物的形成。
在过去的几十年里,炼钢技术的进步导致钢种的杂质含量非常低。近年来,新的“清洁”和“超清洁”钢已被世界各地的钢铁生产商开发并商业化,从而响应了当前和未来市场对具有显着改善机械性能(例如疲劳强度和冲击韧性)的钢材的需求) 和改进的耐腐蚀性。这些钢的氧含量(低于 10 ppm)和硫(低于 10 ppm)含量极低。这些进步背后的驱动力是开发能够承受高要求应用的新钢,例如:用于汽车行业的传动部件,以及用于腐蚀性和腐蚀性环境的建筑零件和管材。
尽管当今的高清洁度钢具有出色的机械性能和/或耐腐蚀性,但这些功能性能的进步是以更难断屑为代价的,在某些情况下,加工操作中的刀具寿命会大大缩短。
高清洁度钢的加工通常与高能耗、增加的刀具磨损和高生产成本相关。据估计,生产汽车部件的总生产成本的 40% 以上来自不同的加工操作。因此,主要问题被评估为在综合机械加工性和性能要求方面优化当今的钢种。因此,夹杂物在某种程度上对于适当的机械加工性能是必需的。但夹杂物的含量和特性仍是保证钢获得高性能性能的关键。
二次炼钢过程的重要特征是生产过程、钢水、钢包耐火材料、添加剂、熔渣、温度以及处理的时间和方法。这些是影响夹杂物各种特性的关键因素,如图1所示。
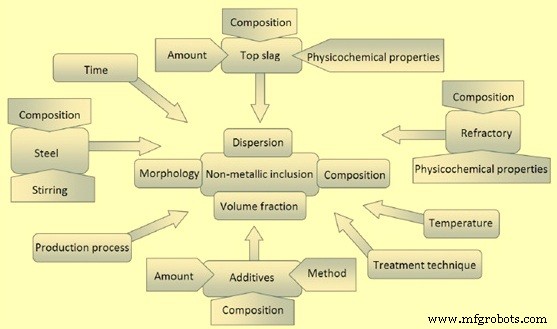
图1 炼钢过程中影响夹杂物各种特性的因素
夹杂物的形成过程分为三个阶段。这些阶段是 (i) 成核,(ii) 生长,和 (iii) 聚结和凝聚。在成核阶段,由于添加剂(脱氧剂或脱硫剂)的溶解,溶液(液态或固态钢)与溶质(例如铝和氧)过饱和,从而形成新相的晶核。或冷却钢。形核过程由边界夹杂物-钢液上的表面张力决定。表面张力越小,形成新相核所需的过饱和度就越低。在钢液中存在其他相(其他夹杂物)的情况下,形核过程要容易得多。在这种情况下,新相的形成取决于核与基质夹杂物之间的润湿角。润湿条件(低润湿角)有利于新相的形核。
在生长阶段,发生细胞核的生长。新夹杂物的生长一直持续到达到化学平衡(没有过饱和)。固体钢中夹杂物的生长是一个非常缓慢的过程,因此可以保持一定程度的非平衡过饱和。
聚结和团聚是由于液体由于热对流或强制搅拌引起的运动导致夹杂物碰撞,这可能导致它们聚结(液体夹杂物合并)或聚结(固体夹杂物合并)。聚结/团聚过程是由夹杂物和钢水之间的界面减少而获得的能量优势驱动的。表面能较高的夹杂物在碰撞时有较高的合并机会。
从钢液中去除夹杂物涉及到其浮选到钢渣界面、从钢中分离以及随后吸收到渣中。钢中夹杂物浮选的基本机理是斯托克斯浮选定律。使用这个方程,对于 20 微米大小的球形氧化铝夹杂物,估计漂浮 2 米距离的时间约为 120 分钟。该浮选时间随着夹杂物尺寸的增加而减少,并且通过氩气搅拌和随后夹杂物与氩气气泡的附着进一步改善。例如,100 微米大小的氧化铝夹杂物在 5 分钟内浮出。氩气搅拌还通过碰撞和随后的团聚/聚结促进夹杂物生长
大的夹杂物比较小的夹杂物漂浮得更快。大的夹杂物通常是漂浮的,因此,它们很容易从钢中漂浮到渣相中。不那么浮力的较小夹杂物需要更长的时间才能从钢中漂浮。漂浮的夹杂物被熔渣吸收。可以通过适度搅拌来加强漂浮过程。剧烈搅拌导致较大的夹杂物破碎成较小尺寸的夹杂物。气泡在钢液中向上移动也促进了夹杂物的上浮和被熔渣吸收。
夹杂物分类
夹杂物是在高温精炼过程中和/或在凝固过程中析出的钢液中产生的。在钢的高温精炼过程中产生的夹杂物称为一次夹杂物,在凝固过程中产生的夹杂物称为二次夹杂物。一旦在钢中形成夹杂物,夹杂物的大小、数量、成分和形态等特征保持不变或由于钢液中的物理化学反应而发生变化/演变,钢液与周围的炉渣和钢包耐火材料之间,并从变形。根据它们的最终特性,它们可能对铸造过程有害,降低钢的机械性能,并降低钢产品的表面和整体质量。夹杂物的存在决定了钢的纯度,根据化学和矿物含量、稳定性和来源进行分类。
根据传统分类,夹杂物可根据其来源分为两大类。这些类别是 (i) 内源性夹杂物,和 (ii) 外源性夹杂物。由于钢中所含化学物质的溶解度降低,内源性夹杂物通过在液相中析出而形成。这类非金属夹杂物不能从钢中完全消除,但必须严格控制其体积分数和平均粒径的减小,以免引起破坏现象。
相反,外源性夹杂物是夹杂来自熔渣、耐火碎片或上升和覆盖粉末的非金属材料的结果,这些粉末用于保护钢,避免在铸钢过程中粘着。属于此类的夹杂物可以具有大尺寸特征,并且它们的来源不能立即识别,尽管它们的存在会严重损害钢的微观结构健全性和相关的机械可靠性。由于外源性夹杂物总是与工艺相关,因此可以通过实施合适的加工程序来消除它们。
内生夹杂物 – 内源性夹杂物(也称为原生夹杂物)出现在钢液中,在冷却和凝固过程中析出。属于此类的夹杂物来自钢中的添加剂。它们是钢冷却和凝固过程中的脱氧产物或析出的夹杂物。 LCAK(低碳铝镇静)钢中的氧化铝(Al2O3)夹杂物和硅镇静钢中的二氧化硅(SiO2)夹杂物是溶解氧与添加的铝和硅脱氧剂反应生成的典型脱氧夹杂物。
氧化铝夹杂物在高氧环境中形成时呈树枝状(图 2)。脱氧或再氧化产生的簇状氧化铝夹杂物(图 2)是典型的铝镇静钢。氧化铝夹杂物由于界面能高,容易通过碰撞和聚集形成三维团簇。簇中的单个夹杂物的直径可以是 1 微米到 5 微米。在与其他颗粒碰撞、破碎或聚集之前,它们可以呈花盘状或(聚集)多面体包裹体。或者,珊瑚状氧化铝夹杂物被认为是由原始树枝状或簇状氧化铝夹杂物的“奥斯特瓦尔德熟化”产生的。由于在钢水中处于液态或玻璃态,二氧化硅夹杂物通常呈球形。二氧化硅也可以聚集成簇。
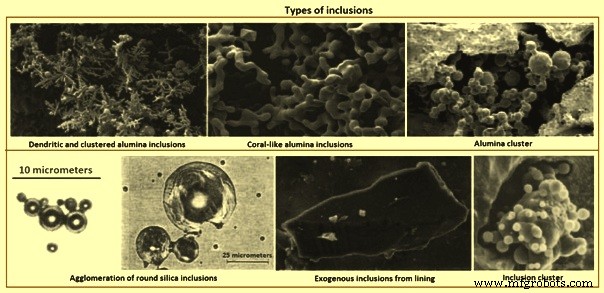
图 2 夹杂物类型
在钢的冷却和凝固过程中会形成沉淀的夹杂物。在冷却过程中,液体中溶解的氧/氮/硫的浓度变大,而这些元素的溶解度降低。因此,诸如氧化铝、二氧化硅、氮化铝和硫化物的夹杂物沉淀。硫化物在凝固过程中在枝晶间形成,并经常在钢水中已经存在的氧化物上成核。这些夹杂物通常很小(小于 10 微米)。
内源性夹杂物通常比外源性夹杂物分布更均匀,外源性夹杂物是来自与钢水接触的耐火材料界面、熔渣或其他材料的截留物。内源性夹杂物是天然存在的,因此只能尽量减少,不能完全消除。氧化铝和镁尖晶石等初级和内源性氧化物堵塞了浸入式水口,它们的不规则形状在变形过程中充当应力上升器,降低钢的机械强度。
外来夹杂物 – 外源性夹杂物是由液态钢与其周围环境的无意化学和机械相互作用产生的。由于它们的大尺寸和靠近表面的位置,它们通常对断裂敏感的机械性能有害。这些夹杂物大部分是通过再氧化形成的,其中溶解在钢水中的“游离”脱氧剂(铝、硅、锰或钙)在浇注和通过浇口运输过程中从与空气的接触中吸收氧气系统。此外,钢水与预热不当的输送容器中蒸发的气体或水发生反应,可能会形成夹杂物。外源夹杂物通常在出钢、浇注和凝固过程中意外夹带,导致在整个铸钢产品中随机分布。这些夹杂物在钢水运动过程中作为异质形核位点析出新的夹杂物。
外源性夹杂物主要来自钢液与周围环境的偶然化学反应(再氧化)和机械相互作用(夹渣和内衬耐火材料的侵蚀)。在加工过程中,它们会产生颤振,造成加工部分表面出现凹坑和凿痕,经常出现破损,以及刀具过度磨损。外源性夹杂物具有以下共同特点。
- 耐火侵蚀夹杂物的尺寸通常大于夹渣夹杂物。
- 复合成分/多相是由以下现象引起的:(i)由于钢液与二氧化硅、FeO和MnO之间的反应,在炉渣和内衬耐火材料中生成的氧化铝夹杂物可以留在其表面,(ii)当外源夹杂物移动时,由于它们的尺寸较大,它们可以在其表面夹带脱氧夹杂物,例如氧化铝,(iii) 外源夹杂物在钢水运动过程中充当异质核位点,用于析出新的夹杂物,以及 (iv)熔渣或再氧化夹杂物会与内衬耐火材料发生反应,或将其他材料移入钢中。
- 不规则形状,如果不是球形,则来自夹渣或脱氧产物二氧化硅。球形外源夹杂物通常较大(大于50微米)且多为多相,而球形脱氧夹杂物通常较小且为单相。
- 与小夹杂物相比,数量少
- 在钢中呈零星分布,作为小夹杂物分散不充分。由于它们通常在浇注和凝固过程中被困在钢中,因此它们的发生是偶然的和零星的。另一方面,它们很容易浮出,因此只集中在钢型材中凝固最快的区域或在某种程度上阻碍它们通过浮选逸出的区域。因此,它们经常出现在地表附近。
- 由于尺寸较大,因此比小夹杂物对钢材性能的危害更大。
超越这些夹杂物来源的一个问题是,为什么如此大的夹杂物一旦形成就不会迅速浮出。可能的原因是 (i) 炼钢过程中的晚期形成、转移或冶金容器中的腐蚀,使它们在进入铸件之前没有足够的时间上升,(ii) 缺乏足够的过热度,以及 (iii) 凝固过程中的流体流动导致结晶器渣浮起的夹杂物在完全进入熔渣之前被夹带或重新夹带。
外源性夹杂物往往与实践有关,其大小和化学成分经常导致对其来源的识别,其来源主要是再氧化、夹渣、内衬侵蚀和化学反应。
图 2 显示了钢中最常见的大宏观夹杂物形式,例如氧化铝簇。空气是最常见的再氧化来源,它可以通过多种方式发生,例如 (i) 中间包混合物中的液态钢由于强烈的湍流,在浇注开始时顶部表面有空气,流动液体表面的氧化膜折叠到液体中,形成薄弱的氧化物颗粒平面,(ii)空气被吸入钢液中钢包与中间包之间、中间包与模具之间的连接处,(iii)浇注时空气从钢包、中间包和模具中的钢的顶面渗入钢中。
在这种再氧化过程中,铝、钙、硅等脱氧元素被优先氧化,其产物发展为夹杂物,通常比脱氧夹杂物大一到两个数量级。防止这种再氧化的解决方案是通过以下方法限制空气暴露在铸造过程中:(i) 利用钢环歧管或多孔耐火环围绕钢包和中间包之间的连接处以及钢包和中间包之间的连接处用惰性气幕覆盖。中间包和结晶器,(ii) 在浇注前将一些气体吹入中间包,并在浇注过程中吹入中间包表面,(iii) 控制钢包内的气体注入以避免形成眼眼。
另一个再氧化源是炉渣和内衬耐火材料中的二氧化硅、氧化锰和 FeO。通过这种再氧化机制,钢中的夹杂物通过 SiO2 / MnO / FeO+[Al] =[Si] / [Mn] / [Fe]+Al2O3 反应在靠近炉渣或炉衬界面处生长。该反应导致具有可变成分的较大氧化铝夹杂物。这种现象进一步以两种方式影响外源夹杂物,即(i)这种反应会侵蚀和使衬里表面不均匀,从而改变衬里壁附近的流体流动模式,并可能导致衬里进一步加速破裂,(ii)大的外生夹杂物破碎衬里或夹带的熔渣可以夹带小夹杂物,如脱氧产物,也可作为新析出物的异质核。这使得外源性夹杂物的成分复杂化。
为了防止炉渣和内衬耐火材料的再氧化,保持较低的 SiO2、MnO 和 FeO 含量非常重要。据报道,游离二氧化硅含量低的高铝或氧化锆砖更适合。
炼钢或转移操作涉及炉渣和金属的湍流混合,特别是在容器之间的转移过程中,因此会产生悬浮在钢中的炉渣颗粒。夹渣,(尺寸为 10 微米至 300 微米,含有大量的 CaO 或 MgO,在钢水温度下通常为液态,因此呈球形。使用“H 形”中间包并通过两个钢包在更换钢包期间减少夹渣。在连铸过程中影响夹渣进入钢水的因素是(i)从钢包到中间包和从中间包到结晶器的转移操作,特别是对于开口浇注,(ii)涡流在当钢水处于低位时,钢水顶面出现涡流可以通过几种方式避免,例如在涡流开始之前关闭浇注,(iii)在顶面乳化和夹渣,特别是在上面的气体搅拌下临界气体流速,(iv) 结晶器弯液面处的湍流,以及 (v) 熔渣特性,例如界面张力和熔渣粘度。例如,结晶器熔渣可以被夹带到液体中由于 (i) 弯液面的湍流,(ii) 涡流,(iii) 气泡从钢中移动到熔渣引起的乳化,(iv) 由于压力差沿喷嘴壁吸入,(v)高速流从表面剪切熔渣,以及(vi)液位波动。
钢与液态铸粉之间的界面张力决定了钢弯月面的高度,以及助熔剂夹带的难易程度。具体而言,对于与纯铁接触的石灰-二氧化硅-氧化铝渣,1.4 N/m 的界面张力产生约 8 mm 的弯液面高度。通过表面活性物质(例如硫)或通过界面交换反应(例如钢中的铝被炉渣中的氧化铁氧化),界面张力降低到低值。与化学反应相关的非常低的界面张力可以通过马兰戈尼效应在界面处提供自发湍流。这种湍流会在界面处形成乳液,从而在钢中产生不希望的渣珠。
耐火材料的侵蚀,包括井块砂、松散的污垢、破碎的耐火砖砌体和陶瓷内衬颗粒,是大外源夹杂物的常见来源,这些夹杂物通常是固体,与钢包和中间包本身的材料有关。它们通常较大且形状不规则。 外源性夹杂物可以作为氧化铝异相成核的位置,并且可以包括图中的中心颗粒、 或与其他土著内含物聚合。耐火侵蚀产物或机械引入的夹杂物的出现会完全损害原本非常干净的钢的质量。
内衬腐蚀通常发生在湍流区域,特别是在再氧化、高浇注温度和化学反应结合时。下面给出对衬砌侵蚀影响较大的参数。
- 某些钢种具有很强的腐蚀性(例如高锰和几乎不被杀死且具有高可溶性氧含量的钢种)并腐蚀衬砖。
- 再氧化反应,例如钢液中溶解的铝减少了衬里耐火材料中的 SiO2,生成了非常活泼的 FeO 基夹杂物并润湿了衬里材料,导致在高流体湍流区域内衬里耐火材料受到侵蚀。这种反应的程度可以通过监测钢水的硅含量来量化。当耐火材料中的碳与粘合剂和杂质发生反应时,这种氧气也可能来自一氧化碳。
- 砖的成分和质量对钢材质量有显着影响。某钢厂在渣线处采用了三种材料(高 Al2O3、Al2O3-SiC-C 和 MgO-C,磨损率分别为 1.0、0.34、0.16 毫米/热),耐火材料容易损坏通过中间包熔剂和熔渣的侵蚀,MgO-C砖在三者中表现出最高的耐久性。氧化锰优先侵蚀耐火材料的含二氧化硅部分。纯度极高的氧化铝和氧化锆晶粒可以承受氧化锰的侵蚀。
- 高锰钢的耐火材料快速腐蚀可以通过以下方法来限制:(i) 使用纯度非常高(昂贵)的氧化铝或氧化锆耐火材料,以及 (ii) 通过使用强脱氧剂(例如铝或钙)完全杀死钢来最大限度地减少氧气,并防止空气吸收。二氧化硅基中间包内衬比氧化镁基喷涂内衬差。高铝耐火材料被认为是最有前途的。将氧化钙加入到喷嘴耐火材料中有助于液化管壁处的氧化铝夹杂物,只要氧化钙扩散到界面的速度足够快并且喷嘴腐蚀不成问题。可以通过控制喷嘴耐火材料成分(例如避免钠、钾和硅杂质)或用纯氧化铝、氮化硼或其他耐腐蚀材料涂覆喷嘴壁来对抗喷嘴腐蚀。应选择护罩壁表面的耐火材料,以尽量减少与钢的反应,从而产生夹杂物和堵塞。
- 钢水沿中间包内壁(例如入口区)的速度过快。垫可以用来防止中间包底部的侵蚀,以及控制流动模式。有人建议,超过 1 m/s 的钢水速度对于腐蚀是危险的。
- 接触或填充时间过长以及高温会加剧侵蚀问题。 During long holding period in the ladle, the larger inclusions can float out into the ladle slag. However the longer the steel is in contact with the ladle lining, the more tendency is there for the ladle erosion products. Solutions are based upon developing highly stable refractories for a given steel grade, developing dense wear resistant refractory inserts for high flow areas and preventing reoxidation.
Chemical reactions produce oxides from inclusion modification when calcium treatment is improperly performed. Identifying the source is not always easy, as for example, inclusions containing calcium oxide can also originate from entrained slag.
The agglomeration of solid inclusions can occur on any surface aided by surface tension effects, including on refractory and bubble surfaces. The high contact angle of alumina in liquid steel encourages an inclusion to attach itself to refractory in order to minimize contact with steel. High temperatures of 1,530 deg C enable sintering of alumina to occur. Large contact angle and larger inclusion size favour the agglomeration of inclusions. Due to the collision and agglomeration, inclusions in steel tend to grow with increasing time and temperature. The numerical simulation of inclusion nucleation starting from deoxidant addition and growth by collision and diffusion from nano-size to micro-size is reported. The fundamentals of alumina sintering into clusters, needs further investigation, though some studies have used fractal theory to describe the cluster morphology (features).
Another classification of the inclusions can be based on their chemical composition. The inclusions can be synthetically classified as (i) sulphides, (ii) aluminates, (iii) silicates, (iv) oxides, (v) nitrides, and (vi) complex combinations of two or more of these inclusion types. The majority of the inclusions in steels are oxides and sulphides since the content of phosphorus is very small. Silicates are very detrimental to steels, especially if it has to undergo heat treatment at a later stage. Normally nitrides are present in special steels which contain an element with a high affinity to nitrogen.
Sulphides inclusions are FeS, MnS, Al2S3, CaS, MgS, Zr2S, and others. The sulphides are frequently the consequence of the calcium treatment applied in order to modify the oxide inclusions, but the little and finely dispersed CaS highly refractory inclusions can be detrimental for the casting procedure (nozzle clogging) and for the damaging effect on steel. On the contrary the MnS non-metallic inclusions (frequently modified by the combination with CaS) are exploited for improving the cutting tool workability. In this case the MnS non-metallic inclusions are intentionally formed within the metal matrix in order to make the chipping brittle) during the tool working. This role implies that the volume fraction of the inclusions has to be significant and this aspect is the reason that excludes the application of EN10247:2003 for the estimation of the cleanliness of such a class of steels.
Aluminates inclusions normally consist of calcium aluminates obtained after the calcium treatment of the liquid steel. Calcium aluminates are 12CaO.7Al2O3 (C12A7), 3CaO.Al2O3 (C3A), and CaO.Al2O3 (CA) exist in the liquid state, whereas CaO.2Al2O3 (CA2) and CaO.6Al2O3 (CA6) are solid at steelmaking temperatures.
Silicates are present in steel like a glass formed with pure SiO2 or SiO2 with admixture of iron, manganese, chromium, aluminium, and tungsten oxides and also crystalline silicates. Silicates are the biggest group among non-metallic inclusions. In liquid steel non-metallic inclusions are in solid or liquid condition. It depends on the melting temperature.
Oxides inclusions in liquid steel are mostly produced during steel deoxidation but can also result from reoxidation and slag or refractory entrainment in the steel. These inclusions can have, single or multiple phases and compositions, spherical or irregular shape, and are either solid or liquid in the steel depending on their melting temperature.
Oxides inclusions can nucleate homogeneously or heterogeneously. Homogeneous nucleation occurs without the presence of foreign surfaces in the steel while heterogeneous nucleation occurs on foreign surfaces. Sources of foreign surfaces in liquid steel can be entrained materials, the surrounding ladle refractory, pre-existing inclusions, and or undissolved alloys. For the formation of a stable oxide, the absolute contribution of the bulk free energy to the overall energy is to be greater than the interfacial energy and this occurs at a critical oxide size. Inclusions less than this critical size are unstable and re-dissolve into the liquid steel while those which are larger than this size grow. For the heterogeneous nucleation, the presence of an existing surface reduces the critical oxide size and hence, reduces the overall free energy needed for a stable oxide to be produced. Heterogeneous nucleation is more favoured compared to homogeneous nucleation.
Important inclusion characteristics are their size, amount, composition, and morphology. After a stable oxide is produced in the steel, the inclusions grow and can also change their composition due to reactions within the steel and between the steel and surrounding slag and ladle refractory. Oxides inclusion growth occurs by diffusion of oxygen and deoxidant to the inclusion, and by agglomeration and coalescing after collision.
Oxides inclusions are FeO, MnO, Cr2O3, SiO2, Al2O3, TiO2 and others. By mineralogical content, oxide inclusions divide into two main groups namely (i) free oxides such as FeO, MnO, Cr2O3, SiO2 (quartz), Al2O3 (corundum) and others, and (ii) spinels which are compound oxides formed by bivalent and trivalent elements. Ferrites, chromites and aluminates are in this group. The fundamental tool for the description of the chemical composition of the oxide inclusions is the ternary phase diagram (CaO-SiO2-Al2O3), since this is the main system ruling the formation of these non-metallic compounds. This class of non-metallic compounds are formed by the deoxidizing elements added to the liquid steel for removing the oxygen content.
Composition, size, and distribution of precipitated oxides are greatly influenced by the deoxidants, conditions of the liquid steel, and the solidification process. Aluminum is widely accepted as deoxidant in steelmaking process. Its addition is very convenient and it effectively reduces oxygen content in liquid steel to low levels. However, the most of the steel problems can be traced to alumina or Aluminum rich oxides. Solid alumina inclusions in the liquid steel tend to rapid clustering due to their dendritic morphology. The alumina clusters hardly float to the top of the liquid steel because of their high apparent density in view of oxide clusters plus engulfed liquid steel. They are detrimental to the castability and quality of continuously cast steel.
The onset of clogging during the casting process starts when an alumina inclusion attaches to the nozzle wall. Certain types of refractories, especially the graphite-stabilized magnesia refractories, have been reported to promote agglomeration of alumina inclusions. The high contact angle between the alumina inclusions and the steel further promotes the tendency of the inclusions to agglomerate on refractories. In addition, the presence of significant amounts of alumina and MnS inclusions negatively impacts the performance of steel products. In general, oxide inclusions can cause lamellar tearing and degrade the toughness, bendability and ductility of steels.
When aluminum is added to liquid steel for deoxidation, the aluminum reacts with the oxygen to form dendritic alumina inclusions (alumina galaxy). Depending on size, the alumina inclusions formed as a result of deoxidation can be divided into macro-inclusions and micro-inclusions. Partial and complete substitution of titanium, zirconium, and / or rare earth metals for aluminum is increasingly pursued. This is done to improve the castability and the quality of the continuously cast steel through generation of finely dispersed oxides which effectively serve as heterogeneous nucleation sites for transformation and precipitation. Hence, control of the amount, size, composition and distribution of inclusions in steel is of importance.
Nitrides inclusions are ZrN, TiN, AlN, CeN and others which can be found in alloyed steel and has strong nitride generative elements in its content. The nitride generative elements are titanium, aluminum, vanadium, cerium and others. The nitride inclusions are normally formed by titanium nitride (TiN) and perform a detrimental effect worsened by the peculiar edged shape which increases the amplification of the stresses which are developed at the interface between the inclusion and the metal matrix. When TiN is present in large numbers, homogeneously distributed, and in relatively small sizes, they promote the formation of equi-axed grains which improve the mechanical strength of the cast steel. Also, the presence of a specific CaO∙Al2O3∙2SiO2 oxide (Anorthite) in stainless steel 316L has been found to improve the machining tool life. These inclusions when present, act as a lubricant by coating the machining tool tip. They also promote the breaking of machining chips.
Examples of complex combinations of two or more of these inclusion types are FeO·Fe2O3, FeO·Al2O3, FeO·Cr2O3, MgO·Al2O3, 2FeO·SiO2, FeS·FeO, MnS·MnO, Nb(C, N), V(C, N) and others.
Three main mechanisms have been recognized at the origin of the inclusions which are related to the damaging effects played by these non-metallic phases against the metal matrix. These mechanisms consider the inclusions as (i) notching elements which amplify the stress field around the inclusions, (ii) pressurized tanks of gas which progressively migrates into the inclusions generating a stress field around the inclusions, (iii) non-metallic phases which generate a residual stress due to the different thermal expansion coefficient associated to the metal phase and the glassy-ceramic ones.
The first mechanism is associated to a ductile process of crack formation which develops starting from the interface between the inclusions and the steel. The voids are the precursor of cracks and on a macroscopic level the cooperative detrimental effect related to the voids formed by a large number of inclusions produces a decrease of the ultimate tensile strain value. This relation points out that the factors detrimentally influencing the toughness and the macroscopic ductility of the steels are (i) the increase of the volume fraction, (ii) the decrease of the curvature radius, and (iii) the fracture of the non-metallic inclusions.
The coalescence among the nucleated voids is very dangerous since the voids of adjacent inclusions can coalesce to form a large crack, so the formation of elongated strips of inclusions represents an extreme situation. Hence, the inclusions constituted by the brittle ceramic phases which can form elongated fractured strips have to be carefully avoided. It is worth noting that the just described mechanism is featured by a ductile process on microscopic scale, but its effect on a macroscopic level turns out as a decrease of the toughness and of the ductility.
The second mechanism is related to the highest solubility shown for hydrogen by the sulphides. Hence, the inclusions become pressurized tanks pulling on the metal matrix and giving rise to a stress field which can be summed to the one formed by the external force applied during the service of the steel.
The third mechanism takes place as a consequence of the different thermal expansion coefficient featuring the steel and the glassy and / or ceramic structures characterizing the inclusions. The silicates, the aluminates, and normally all the oxides (except CaO and MgO) have a thermal expansion coefficient lower than one of the steel metal matrix, while the sulphides are featured by a contrary behaviour. The detrimental action is due to the residual stress generated on the interface between the inclusions and the metal matrix.
The higher the size of the inclusion the larger is the detrimental effect, so in order to prevent this mechanism the limitation of the size of the inclusion is a fundamental aspect while the overall volume fraction of the inclusion population does not play a significant role in this mechanism.
By stability, non-metallic inclusions are either stable or unstable. Unstable inclusions are those which dissolve in dilute acids (less than 10 % concentration). Unstable inclusions are iron and manganese sulphides and also some free oxides.
The formation and the control of the chemical composition of the inclusions involve the different steps of the production processes and the industrial systems through which they are performed. The production process has to be carefully implemented in each step in order to avoid problems related to (i) difficulties during the casting operation associated with the nozzle clogging between the tundish and the mould (continuous casting process) and between the ladle and the casting column (ingot casting), and (ii) detrimental effect on the mechanical properties of the steel.
There are four main treatment mechanisms for the removal of inclusions from the liquid steel. The first mechanism is the flotation of the inclusions. As per the Stokes law, because of the differences between densities of non-metallic inclusions and liquid steel, flotation leads to the removal of the inclusions. It is possible to calculate theoretically the rate of inclusion removal due to flotation. The second mechanism is the use of the magnetic stirring and argon gas injection. These two techniques assist the removal of non-metallic inclusions. Rate of inclusions entrapment by means of argon gas injection can be calculated.
The third mechanism is the calcium treatment. Calcium treatment is an effective way which can facilitate the removal of inclusions from the liquid steel. By adding calcium to the liquid steel (mostly in form of calcium silicide), it is possible to modify unmelted aluminum-magnesium rich inclusions (spinels) to large, isotropic, and spherical calcium aluminates and calcium sulphides with low melting points. This assists the removal of liquid inclusions. However, it can become a problem if for any reasons some of these large calcium aluminates remain or get trapped in the liquid steel.
The fourth mechanism is to optimize the properties of the top slag. Optimized properties of the top slag can enhance the inclusions removal in the ladle furnace. The three mechanisms mentioned above facilitate the inclusions movement from the middle or bottom parts of the liquid steel bring the inclusions to the ladle top. However without a proper top slag, it is highly probable that these inclusions cannot be removed efficiently. Hence, in order to ensure a very effective entrapment and absorption of non-metallic inclusions by means of top slag, it is necessary to have an optimized liquid top slag with high absorbing capacity for inclusions, proper wetting properties, and viscosity.
Inclusion engineering
Solid-phase inclusions can cluster together to clog nozzles and other flow control systems which mediate the flow of liquid steel, posing a threat to the process operations. Some inclusion chemistries reduce ductility, resistance to fatigue, or overall toughness in steels. The absence of inclusions poses issues as well because the ‘clean steels’ can be harder to machine, decreasing the lifespan of cutting tools, and require higher power consumption for machining. Understanding their nature is of critical importance in steelmaking operations, and ‘inclusion engineering’ is needed to be an operational focus during the process of steelmaking.
The term ‘inclusions engineering’ means the design of the inclusions so as to alleviate their harmful effects on the product properties. Inclusion engineering does not refer to removal of inclusions but it refers to modify them either in terms of chemical composition or shape so that harmful effects of the inclusions can be converted to improve the steel properties. Inclusion engineering also involves distribution of inclusion uniformly in the matrix, so that composite properties can be generated in the product. In some cases, deliberate attempts are made to form very fine inclusions (e.g. nitrides, and carbo-nitrides inclusions in hardening steel). Such inclusion can form by reaction between tungsten, titanium, aluminum with oxygen, nitrogen, sulphur, or carbon.
The approach for reducing the harmful effect of inclusions is to tailor the steelmaking process to avoid the presence of macro-inclusions while controlling the population, size, distribution, and morphology of the residual micro-inclusions in the steel. The application of new technology and the knowledge gained from end users on the performance of steel products are valuable information for use in the design of a clean steel strategy. The science of inclusion modification and shape control stems from the need to change the chemistry of the inclusions to enhance the performance of products in the field and ensure the castability during continuous casting. However, macro-size inclusions are required to be removed. In all other cases, depending on applications, inclusion can be modified to minimize their harmful effects.
As far as inclusion modification and shape control are concerned, the inclusions of interest are the endogenous type, particularly the inclusions which result from the process of deoxidation and sulphide-type inclusions. Oxides and sulphides are the two predominant inclusions in steel. The sources of oxides and sulphides are inherent to the steelmaking process. Oxygen is employed to react with the impurity elements (e.g. silicon, manganese) and carbon to generate chemical energy for the melting process. However, a significant amount of the oxygen ends up being dissolved in the liquid steel. The dissolved oxygen is required to be removed during the refining stage because of its harmful effect on the structural integrity of the finished product. Strong deoxidants, like aluminum and silicon, are normally used to scavenge oxygen from the steel. However, aluminum-killed steels routinely clog tundish well nozzles and submerged entry nozzles during continuous casting due to the residual alumina inclusions which remain in the steel.
The element which is to be added to modify the inclusions is to meet three requirements namely (i) it is to have high chemical affinity for the inclusion, (ii) it is to be able to modify the composition so that it becomes liquid, and (iii) it is to be able to modify the shape i.e. sharp edges and corner of inclusions to spherical.
The formation of the non-metallic phases is ruled by the thermodynamic relations. The oxide system represents the most difficult one to be studied because of the presence of different oxide species.
Moreover, the insertion of calcium aiming at the modification of the inclusions makes even more difficult the understanding of the interaction taking place in the steel bath. A good procedure for the engineering of the inclusions is aimed at developing low melting non-metallic oxides in order to avoid the nozzle clogging and at maintaining a prevalently glassy structure of the inclusions during the steel cooling and the successive heating imposed to perform the plastic deformation in order to avoid the formation of ceramic brittle phases. The need to stabilize the glassy structure makes interesting the formation of silicate system based on the presence of anorthite and pseudo-wollastonite which appears to be particularly favourable.
The prediction and the engineering of the oxide inclusions can be based on a powerful and simple thermodynamic model and can be divided into three main steps namely (i) computation of the oxygen potential associated with the slag, (ii) evaluation of the possibility of the development of the reactions to create some pure non metallic compounds, and (iii) definition of a hierarchy of the different reactions as a function of the associated oxygen potentials on the basis of the chemical composition of the steel.
Ductility is appreciably decreased by increasing amounts of either oxides or sulphides. Fracture toughness decreases when inclusions are present in higher-strength lower-ductility alloys. Similar property degradation from inclusions is observed in tests which reflect slow, rapid, or cyclic strain rates, such as creep, impact, and fatigue testing. Inclusions cause voids, which can induce cracks. Large exogenous inclusions can cause trouble in the form of inferior surface, poor polishability, reduced resistance to corrosion, and in exceptional cases, slag lines and laminations. Inclusions also lower resistance to HIC (hydrogen induced cracks). The source of most fatigue problems in steel are hard and brittle oxides, especially large alumina particles over 30 micrometers. Lowering the amount of large inclusions by lowering the oxygen content to 3 ppm to 6 ppm has extended the life of steel part such as bearing by almost 30 times in comparison with steels with 20 ppm oxygen. To avoid these problems, the size and frequency of detrimental inclusions are to be carefully controlled. Especially there is to be no inclusions in the casting above a critical size.
Although the solidification morphology of inclusions is important in steel castings, the morphology of inclusions in wrought products is largely controlled by their mechanical behaviour during steel processing, i.e., whether they are ‘hard’ or ‘soft’ relative to the steel matrix. The behaviour of different types of inclusions during deformation is schematically illustrated in Fig 3. ‘Stringer’ formation, type (b) and (c), increases the directionality of mechanical properties, adversely affecting the toughness and ductility in particular. The worst inclusions for toughness and ductility, particularly in through thickness direction properties of flat-rolled product, are those deforming with the matrix, like (d) in Fig 3.
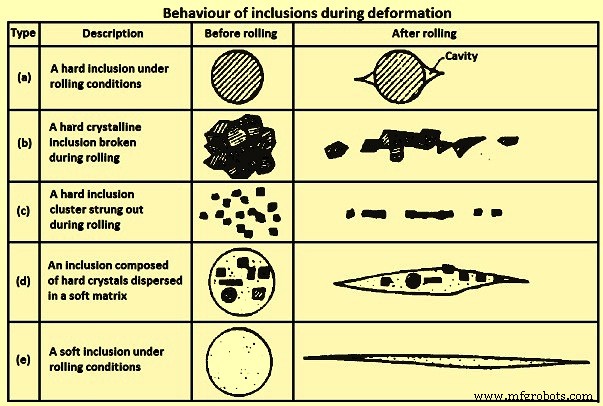
Fig 3 Behaviour of inclusions during deformation
There is a lot of information available on the effect of inclusions on product performance and on the kinetic and thermodynamic phenomena associated with inclusion evolution and formation. With a careful analysis of the available information, it is possible to develop a good practice at each stage of the steelmaking process for clean steel production. However, it is not possible or even necessary to eliminate all inclusions, as certain inclusions which are detrimental to steels for one application can be entirely harmless when present for another application. Hence, steels are expected to have varying degrees of cleanliness depending on their application.
A classification for what has to be considered a macro-inclusion has not been defined in any standard. On the other hand this information can be extremely difficult to be provided, since for a round shape inclusion a diameter of 14 micrometers to 20 micrometers can be dangerous, but for edged inclusions (i.e. TiN) the dangerous size can be stated even at a lower level (2 micrometers to 4 micrometers) as a consequence of the higher stress amplification associated to the edged shape. The treatment of this aspect is further complicated by the fact that the danger level can be strongly affected by the configuration of the non-metallic system which is ruled by the chemical composition of the participating phases. Actually, a correct engineering of the inclusions can permit to realize a sulphide crown precipitated on an oxide core and this system configuration mutually compensates the expansion coefficient of the non- metallic phases, approximating the one of the steel metal matrix.
Calcium treatment – The process of reducing the harmful effect of micro-inclusions by controlling their size, shape, and properties is known as inclusion modification. A common approach to modifying oxide and sulphide inclusions to prevent clogging and minimize any negative effects on the structural integrity of steel is through calcium injection during secondary refining of the steel. Fig 4 gives schematic of inclusion modification with calcium treatment of steel.
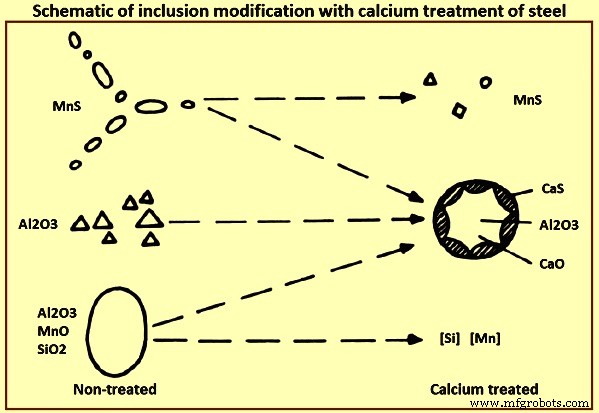
Fig 4 Schematic of inclusion modification with calcium treatment of steel
Calcium has a strong affinity for oxygen and can therefore be used as deoxidizers. However, the use of calcium as deoxidizer is challenged by its low boiling point of 1,439 deg C, limited solubility of 0.032 % of calcium in steel at 1,600 deg C, and a high vapour pressure of 1.81 atmospheres at 1,600 deg C. These properties make it difficult and non-economical to use calcium as deoxidizers. However, combinations of calcium and aluminum or manganese / silicon deoxidation form modified primary inclusions with lower activity and melting temperatures. For this reason, in steelmaking, calcium is added to steel more as an inclusion modifier rather than deoxidizer. Most steel grades are treated with calcium using either Ca-Si alloy or Ca-Fe(Ni) mixture depending on the alloy specification. Normally this treatment is effectively done after trim additions and argon rinsing.
The extent of inclusion modification in steel is an essential feature in secondary steel refining by calcium treatment. Portion of the calcium added to the melt undergoes reaction and remain in the melt as dissolved calcium in form of inclusions or go to the slag as slag constituent. The rest escape the system in form of vapour. It is vital that the calcium added is consumed by the liquid steel to the maximum extent to make the calcium injection efficient and cost effective.
The general effect of calcium treatment on inclusions modifications are (i) manganese sulphides are reduced in number and size, and they are transformed to calcium-manganese sulphides with varying properties, (ii) aluminum oxides, which are normally hard, angular and frequently appears in clusters are reduced in number or completely eliminated and replaced with complex CaO-Al2O3 or CaO-Al2O3-SiO2 inclusions, (iii) silicates are eliminated and replaced by CaO-Al2O3-SiO2 inclusions, and (iv) complex globular CaO-Al2O3-SiO2 inclusions are formed, frequently surrounded by sulphide rim.
Calcium is being frequently employed to treat aluminum killed steels to avoid the formation of solid alumina. Calcium treatment effectively improves the castability and the quality of the continuously cast steel, but is limited for all steel products which need either high fatigue resistance in service or high cold formability in very thin gauges. This is because of the presence of the globular calcium-aluminum oxides. The aluminum, calcium, and calcium-aluminum oxides are normally several to tens of micrometers in diameter.
Rare earth metals like cerium, and lanthanum etc., have also been used to modify inclusions, but they are not as efficient as calcium due to the slow flotation (due to their weight) of the modified inclusions. In addition, lanthanum and cerium readily corrode the ladle refractories. When calcium treatment is efficiently performed, the following two primary objectives are achieved.
- The alumina and silica inclusions are converted to liquid calcium aluminate and calcium silicate, which are globular in shape because of a surface tension effect. This change in inclusion composition and shape is known as inclusion morphology control.
- The calcium aluminate inclusions retained in liquid steel suppress the formation of MnS stringers during solidification of steel. This change in the composition and mode of the precipitation of sulphide inclusions during solidification of steel is known as sulphide morphology or sulphide shape control.
The conversion of inclusions to a globular shape plays a significant role on the separation rate of inclusions. For example, it has been observed that the alumina inclusions are non-wetting in liquid steel and tend to have a higher separation rate compared to CaO-SiO2-Al2O3. This implies that, by modifying the alumina inclusions with calcium, their ability to cluster is impeded as the liquid globular inclusions formed, and as a result are wetted by liquid steel. However, the high vapour pressure of calcium with the associated intense bath stirring promotes collision and coalescence of the alumina inclusions in the liquid steel. With the aid of calcium vapour and the resulting coalescence of the alumina inclusions through collision, their removal from the steel is enhanced compared to the small non-buoyant alumina inclusions which are to first cluster on their own (without forced convection) before they are able to separate from the liquid steel. This is why unmodified small alumina inclusions separate from the liquid steel and get attached to the refractory in the tundish only well after refining is complete in the ladle.
After effective calcium treatment all oxide inclusions normally contain some amount of calcium. Effective modification of oxide inclusions in steel depends on the dissolved aluminium and oxygen content of the steel before calcium treatment. For an essential inclusion modification, a calcium lower limit of 15 ppm to 20 ppm is needed. With a CaO-Al2O3 ratio of 12:7, low melting points of 1,455 deg C of calcium aluminate inclusions are formed. These inclusions exist in the liquid state at steelmaking temperatures.
Agglomeration of alumina, calcium aluminate and CaS inclusions on tundish nozzle refractories during continuous casting can result in a premature termination of casting due to a completely clogged nozzle. Depending on the population of the inclusions in the steel, complete clogging of the nozzle can occur within minutes of the start of casting. Analysis of clogged material in the tundish nozzle typically shows the presence of solid calcium aluminate inclusions with composition rich in either Al2O3 or CaO. For avoiding clogging during continuous casting, it is important to ensure low oxygen potential is achieved during refining prior to calcium treatment. The castability of steel has been shown to be directly related to its oxygen content.
When the calcium treatment is effective, alumina inclusions are converted to molten calcium aluminates which are globular in shape. The calcium aluminate inclusions retained in the steel suppress the formation of harmful MnS inclusions during the solidification of steel by modifying MnS inclusions to spherical CaS inclusions. When alumina is modified to calcium aluminate, the reaction sequence with additional calcium additions is Al2O3 to CA6 to CA2 to CA to C12A7. The presence of liquid calcium aluminates, CA2, CA, C12A7 at steelmaking temperatures (around 1,600 deg C) results in inclusions which are much easier to float than the solid alumina inclusions and also reduce the tendency of blocking ladle and casting nozzles.
The practice is to introduce calcium-bearing agents (CaSi, CaFe, CaAl, CaC, etc.) into the steel at the end of the steel refining in the form of powder or wire injection through hollow metallic tubes. Irrespective of the calcium bearing agent employed, the quantity of calcium required for treatment in a given weight of steel depends on the alumina content, and the oxygen and sulphur levels of the steel. A sufficient amount of calcium is required to be added to react with the alumina inclusions to form calcium aluminate compounds which are liquid at steelmaking temperatures. For completely modified inclusions, the equilibrium reactions are (i) [Ca] + [O] =(CaO), (ii) [Ca] + [S] =(CaS), (iii) 7(Al2O3) + 12[Ca] + 12[O] =12CaO·7Al2O3, and (iv) [MnS] + 2[O] + CaSi =(CaS) + (SiO2) + [Mn].
The reaction in equation (iv) for the precipitation of MnS in the bulk of the liquid steel is possible in steel containing a high sulphur level. Fig 5 shows the binary phase diagram of CaO-Al2O3. The highlighted region in the figure shows the desirable composition of the calcium aluminate inclusions. Outside the highlighted region, the phases are solid at steelmaking temperatures. These phases can be the prominent constituents when there is an over- injection or under-injection of calcium. While MnS inclusions are undesirable in the steel, the formation of solid CaS inclusions is equally undesirable. In terms of clogging, solid calcium aluminate or pure CaS inclusions are just as detrimental as the alumina inclusions. They also sinter and agglomerate on nozzle refractories.
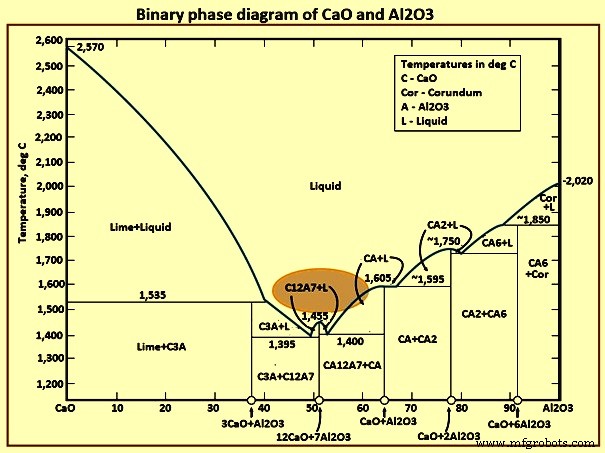
Fig 5 Binary phase diagram of CaO and Al2O3
The efficiency of calcium treatment is dependent on a number of factors, including the type, the amount and the injection rate of the calcium-bearing agent used for the treatment. Overall, by classifying the alumina and MnS inclusions according to their compositions and shapes, the efficiency of calcium treatment can be evaluated as given below.
- Class A inclusions are present when high levels of calcium have been added to the liquid steel and are liquid throughout processing. The intermingled sulphide and aluminate phases of these inclusions indicate that both phases solidified at about the same time. The sulphide phase tends to be a CaS composition. The calcium aluminate phase is either CaO·Al2O3 or 12CaO·7Al2O3. This indicates the presence of calcium aluminates with the lowest melting points and with high levels of calcium.
- Class B inclusions are the ‘bulls-eye’ type most prevalent in calcium-treated steels. The central, dark aluminate phase has solidified first, and then the outer sulphide phase precipitated onto it. In this instance, the sulphide phase tends to be (Ca, Mn)S. The calcium aluminate is of the CaO·Al2O3 or CaO·2Al2O3 composition.
- Class C inclusions are indicative of incomplete calcium treatment. These inclusions have an unmodified MnS phase, which is deformable during hot rolling. The central, dark calcium aluminate tends to be of the CaO·6Al2O3 composition, which has the lowest calcium content and remains undeformed during hot rolling.
- Class D inclusions are alumina-like oxide inclusion clusters which can have some calcium associated with them. However, there is not enough calcium present to result in complete fluxing of the alumina galaxy.
- Class E inclusions are MnS inclusions which are present when sulphur has not been completely tied up by calcium.
- Class F inclusions are inter-dendritic MnS inclusions which are present when sulphur is not completely tied up by calcium and the oxygen potential of the steel is high.
The end results of an optimized calcium treatment are:(a) the alumina is modified to form liquid calcium aluminate, and sulphur is tied up as CaS, which precipitates on the calcium aluminate inclusions, and (b) flotation of the inclusions is improved through the formation and agglomeration of spherical oxide and sulphide inclusions.
Several studies have attempted to determine the required amount of calcium addition for optimal cleanliness. For example, Ca/S ratios have been correlated to reduction of area in the Z direction and impact properties of steel. This approach cannot be generalized to all levels of sulphur. The acceptable level of Ca/S ratio in steels containing low sulphur levels can be several times higher than in steels containing higher sulphur levels, although the absolute amounts of calcium additions in the low-sulphur-containing steels are less than those of the steels containing higher sulphur levels. A good refining practice in the ladle and an efficient calcium treatment results in the majority of the alumina inclusions being converted to liquid calcium aluminate while most of the sulphur is tied up as CaS. The CaS precipitates on the calcium aluminate to produce the desirable bulls-eye shape.
Improvements of steel properties have been reported for calcium treated steel. These include (i) improvement of mechanical properties especially in transversal and through thickness direction by modifying MnS to undeformed globular (Ca-Mn)S or CaS, (ii) improvement of steel machinability at high cutting by forming protective film on the tool surface that prolongs the life of the carbide tool, (iii) improvement of surface quality and polishability, (iv) minimizing lamellar tearing in large restrained welded structures and the susceptibility of steel to reheat cracking as in the heat affected zones (HAZ) of welds, and (v) improvement of steel castability by preventing or minimizing nozzle clogging.
制造工艺


