了解高炉喷煤
了解高炉喷煤
煤粉喷射 (PCI) 是在高炉 (BF) 中生产铁水 (HM) 的成熟技术。大多数 BF 都采用了这种方法,并且所有新的 BF 通常都具有 PCI 功能。用于喷射的煤的成分和性质会影响高炉的运行、稳定性和生产率、高炉的质量以及高炉气体的成分。用于 PCI 的煤炭在链接“http://www.ispatguru.com/coal-for-pulverized-coal-injection-in-blast-furnace/”下的文章中进行了描述。
PCI 系统的关键方面包括煤的制备、储存和分配,以确保煤均匀地输送到每个风口,而不会影响煤的输送速度,以及通过喷枪设计和氧气 (O2) 喷射来燃烧。
煤炭制备
根据需要,煤的粉碎在单个或多个磨机(粉碎机)中进行。煤的研磨和分配到喷枪构成了主要的运营成本。从煤库中回收的煤经过筛选以去除异物,并将任何大块煤粉碎。然后将煤送入磨粉机,在那里进行粉碎和干燥。所需尺寸的煤由热气流运出磨机,收集在袋式过滤器中并输送到储料仓。研磨和运输在惰性气氛下进行,以尽量减少干煤颗粒着火的风险。煤粉的粒度分布会影响其在气动运输设备中的可操作性,以及在高喷射率下的可燃性。
粉碎机将煤研磨成两种尺寸部分之一,即 (i) 煤粉,其中约 70% 至 80% 的煤在 75 微米(微米)以下,其余的在 2 毫米以下,以及 (ii) 具有顶部尺寸为 2 毫米至 3 毫米,2% 的煤超过 2 毫米,20% 至 30% 的煤低于 75 微米。喷射这种煤尺寸的系统称为粒状煤喷射 (GCI)。较粗的研磨具有较低的研磨和干燥成本的优点,并且研磨的煤更容易处理。更精细的磨削在滚道中具有更高的烧损。 PCI 是比 GCI 更受欢迎的技术。
可通过多种措施改变粉碎机中的煤细度,包括改变煤的进料速度、分级器设置或空气流速。尽管可以调整磨机以适应特定的煤以生产所需的尺寸,但这在使用大量煤的混合煤的情况下是不切实际的。在这种情况下,某些煤成分可能达不到要求的细度。
粉碎机的功能之一是从煤中去除尽可能多的水分。干燥是必要的,因为水分会导致气动运输系统和存储箱中的自由流动问题。此外,需要将水分降至最低,因为在 BF 中去除水分需要额外的能量,并且水分的注入会增加还原剂的速率。此外,水分含量高的煤在粉碎过程中会消耗更多的粉煤机功率,从而降低产量。
煤表面水分的蒸发防止了粉碎机内的结块问题,因为具有高水分和粘土含量的煤大多易于粘附。因此,通常需要将煤的总水分含量降低到平衡水分水平附近,以减少磨煤机和储料仓内的处理问题。离开磨机的煤的水分含量应为平衡水分水平的三分之二。煤的平衡水分随煤级、煤质组成和灰分含量而变化。
为了降低运营成本,重要的是要确保将煤研磨到所需的细度,同时使粉碎机部件的磨损最小并且功耗最小。磨损会影响粉碎机的停机和维护。影响磨损的煤特性包括灰分含量和成分、粒度分布、水分和堆积密度。由于磨损和腐蚀的综合影响,煤的较高水分含量会加速磨损。煤灰中的磨料(硬质)矿物包括二氧化硅 (SiO2) 和黄铁矿 (FeS2)。除了研磨元件的磨损外,磨料矿物还会腐蚀管道和管道。评估煤的磨损性能最常用的测试是“磨损指数”(AI)。通常,具有高 AI 的煤预计会导致更高的磨损率。
磨机能耗的降低降低了运营成本。磨机的功耗和产能(生产量)取决于其设计、磨机设置、所需的细度和煤的特性。所需的煤尺寸减少得越高,所需的电力消耗就越大。更高的煤细度需要增加磨机容量,这在研磨难处理的煤时可能也是必要的。
对磨机耗电量和产能有主要影响的主要煤特性是硬度,由“Hardgrove 磨削指数”(HGI) 确定。通常,HGI 越高,磨煤越容易,从而降低功耗和提高产量。如果粉碎机的设计能力限制了 PCI 速率,则可以通过改用较软的煤来提高喷射速率。增加低挥发性物质 (VM)、高热值 (CV) 软煤在高 VM、硬煤混合中的百分比有助于提高粉碎机容量,以及降低高炉中的鼓风压力和改善煤耗。
宏观成分也影响研磨。一般来说,较高的镜质体煤往往比较低的镜质体煤具有较低的研磨能量需求,因为镜质体比惰性和褐煤更容易研磨。当镜质体和惰性体所需的断裂能大致相同时,秩的影响在 1.6 左右的反射率以上降低。
通常混合煤以优化相对强度。然而,混合物并不表现为其组分的平均值,而是可能受到具有问题特征的一种煤的不成比例的影响。当 HGI 相差超过 20 的两种煤的混合物被粉碎时,会优先研磨较软的煤。 “硬”和“软”煤混合物的粉碎表明,成分煤的不良特性往往主导混合物,粉碎机的性能更接近于较硬的煤。研磨共混物时也会优先研磨较软的微晶。含有膨胀粘土的煤在离开粉碎机并冷却后会吸收水分。即使作为混合物的成分存在,此类煤也会导致喷射系统堵塞。
喷煤系统
喷射系统通过喷射容器将煤粉从储仓中气动输送并计量,在喷射容器中,煤粉被加压至或高于 BF 压力,然后到达风口喷射喷枪。喷枪通过风口等量喷射煤,风口围绕高炉圆周对称布置。分配系统设计中的一个关键因素是确保向每个风口均匀供煤,而不会出现输煤路线的波动。煤炭供应的任何中断都会很快导致严重的问题。注入率越高,意外中断的后果就越严重。
至少需要两个喷射容器来为高炉提供连续的煤流。基本上,这些容器有两种不同的布置方式,即(i)串联布置,上部容器定期补充下部容器,始终保持压力并注入煤,以及(ii)并行布置,两个容器交替注入在转换期间进行重叠操作以保持喷煤。
控制喷煤量很重要。因此,喷射容器被连续称重,煤的流量被仔细控制。煤粉在为注入容器和管道供料的储存仓中的处理问题是由于水分和超细颗粒的量,以及煤中存在粘土。可能需要外部加热器和/或绝缘材料来降低由于箱壁内部可能发生的水分凝结而导致箱堵塞的可能性。有的地方通过中喷罐底部的曝气垫吹入氮气(N2),保证煤粉输送到下喷罐时自由流动。
来自注入容器的煤通常通过 (i) 单独的管道输送到每个风口,在这种情况下,煤的数量是独立控制的,并在每个管道中装入,(ii) 一条公共管道到靠近高炉的分配器,在这种情况下,分配器将煤平均分配到通向每个风口的单独管道中。该系统的一个优点是选煤厂和高炉之间的距离可以比单独的管道系统更长。
管道到风口的路径不同以及煤在分流点处不可避免的不均匀分流会导致向风口的进料不均匀。不平衡也会导致管道和分配器的不均匀磨损。
根据煤与输送气体的比例,煤以稀相或密相从喷射容器气动输送到风口。煤稀相系统的输送气体负荷通常为每公斤载气10公斤煤左右,载气速度为15米/秒(m/s)至20m/s左右。载气通常是 N2 和空气与压缩空气的混合物,被添加到注入容器下方的管道中。在密相系统的情况下,每公斤载气的装载量约为40公斤至80公斤煤,载气速度约为1m/s至5m/s。载气一般为N2或N2和空气的混合物。
载气速度应始终高于最小输送速度,以防止堵塞。这个最小速度取决于许多参数,包括系统压力和管道直径。这些变量相互影响。密相系统的低速度意味着管道和部件磨损低,而稀相系统的高输送速度会导致磨损,特别是在弯管处。磨损率由煤颗粒的硬度、形状和速度决定。煤的特性也会影响磨损。用例如聚氨酯弹性体材料对管道易磨损的部分进行衬里可提供耐磨性,并防止可能导致堵塞的细粒堆积。与输送管线堵塞有关的煤质主要有水分和粘土矿物。
煤和混合物中的高水分会产生问题。因此,对磨碎的煤应用了严格的水分限制。粘土的存在会在水的存在下膨胀,这可能会导致问题,尤其是在输送系统中存在压降和/或存在超细颗粒的情况下。随着煤粉中细粉含量(小于 5.8 微米)的增加,输送系统中的压降也会增加。如果压降超过与工厂设计相关的某个值,则可能会发生堵塞。管道堵塞是由于管道弯头处沉积物的堆积,这通常与煤的软性有关(较细的粒度分布)。在注入煤的同时,超细煤(小于 10 微米)通过粘附在弯管壁上启动该过程,一旦形成粗糙表面,则较大的颗粒开始粘附。此外,混合煤中较软的煤优先研磨会导致超细颗粒比例过高,从而导致堵塞。
可以通过改进管道布局和分配系统来防止堵塞,在某些情况下,可以通过调整制备系统(例如磨煤机)以产生更粗的粒度。喷射系统通常具有检测和清除堵塞的程序,因为这是一种常见现象。输送线包括清除堵塞物的吹扫口,通常使用高压空气。需要一个简单实用的测试来评估煤粉和煤混合物的流动性和可操作性。这样可以在使用之前识别困难的材料。
喷枪将煤喷入通向风口的吹管中。颗粒立即被热风加热、点燃、气化和燃烧。喷枪的设计和布置影响煤的燃烧效率。早期的喷枪曾经是直钢喷枪,位于或靠近风口/吹管接口处。已经开发了将 O2 直接注入煤颗粒流(氧煤喷枪)和/或在喷枪尖端产生更多湍流的方法的设计,以提高燃烧效率。这些包括 (i) 同轴喷枪(煤通过内管喷入,O2 通过周围环空喷入),(ii) 高分散喷枪,(iii) 斜面喷枪,(iv) 狭缝喷枪,(v) 偏心(非-同心)双喷枪,和(vi)旋流喷枪。
还实施了煤的预热以提高燃烧效率。最初引入煤时出现的问题,例如喷枪和风口堵塞以及喷枪尖端熔化,已在很大程度上得到解决。堵塞主要是由于煤被加热到一定温度而变得粘稠并粘附在喷枪和风口的表面上。通过使用具有高灰熔融温度 (AFT) 的煤,可以最大限度地减少灰沉积。出于所有实际目的,AFT 应比热风温度高 50 摄氏度。如果煤在风口尖端附近流动性很高,喷枪也会堵塞。这可以通过避免使用具有高粘结指数的煤,或通过增加流量来克服。
将喷枪定位在更靠近风口的位置可以减少灰烬在吹管中的冲击程度。使用风冷同轴喷枪有助于防止堵塞和腐蚀,并可以延长尖端的使用寿命。应尽量减少冷却空气的流量,以降低其对煤燃烧的冷却效果。然而,喷枪堵塞仍然经常发生。有既定的程序可以在它们引起任何问题之前检测和清除这些阻塞。
喷枪使用不同合金和限制热风温度也影响了喷枪尖端的熔化。喷枪的耐用性是一个重要的操作考虑因素,因为它会随着时间的推移而燃烧。
煤炭燃烧
滚道是高炉的重要区域,尽管它们的总体积通常不超过高炉内部体积的 1%。它们为该过程提供热量和还原剂。喷煤不可避免地会影响滚道条件,进而对滚道外产生影响。离开滚道的未燃烧颗粒可能会导致操作问题,例如渗透性降低、气体和温度分布不理想、焦炭侵蚀过度以及炭携带量增加。未燃烧炭的数量随着注入速率的增加而增加。因此,喷吹煤在滚道中的燃烧和气化行为是高炉稳定运行的重要因素。很明显,高炉比在滚道内燃烧的煤消耗更多的喷射煤,因为未燃烧的材料在炉子的其他地方被消耗。
高炉内的煤燃烧已被广泛研究。这些研究是使用台式设备进行的,例如热重分析 (TGA)、滴管式炉 (DTF) 和金属丝网反应器 (WMR)。这些技术不能完全模拟滚道内的条件。例如,煤粉颗粒在 DTF 中的停留时间约为秒,而在 BF 的滚道中约为毫秒。因此,这些技术通常用于对不同类型的煤进行比较评估。
遵循的另一种方法是使用专门设计的设施来模拟跑道状况。其中包括将热风喷射到填充焦炭床中,通常称为“热模型”。它们能够模拟毫秒的短停留时间以及不同的滚道位置的燃烧条件。然而,中试规模的设施仍然不能完全模拟高炉中的滚道条件。例如,它们可能无法在接近风口/鼓风主管压力的压力下工作。较高的滚道压力会提高煤气化率。
许多计算机模型可用于评估煤在滚道和高炉其他地方的行为。这些模型的有效性受到质疑,因为它们所描绘的机制很复杂并且没有被完全理解。它们的准确性取决于所做的假设和模型中建立的关系的有效性。由于煤的行为受到高炉设计和操作条件以及煤特性的强烈影响,因此计算机模型可能仅适用于特定的高炉、操作条件和已开发和开发的相同类型的煤。测试。这些是所有这些技术的局限性。
喷枪出口和滚道后壁之间的煤燃烧(物理距离约为 0.7 m 至 2 m)发生在高温(1400 摄氏度至 2200 摄氏度)、高压(约 3 kg/ sq cm 至 6 kg/sq cm 和短的停留时间(粉状颗粒为 10 毫秒至 40 毫秒)。正是在这些严酷的条件下,需要实现高水平的煤燃烧。
煤的燃烧过程可以分为以下几个步骤,其中一些步骤是重叠的。
- 注入的粉状颗粒(小于 75 微米)在进入富氧热风时会被迅速加热。加热速率由操作条件决定,但约为每秒 100 摄氏度。热风温度通常为 1000 摄氏度至 1200 摄氏度,气体速度约为 180 m/s 至 250 m/s。
- 颗粒发生热解以产生不可冷凝的挥发物(气体)、可冷凝的挥发物(焦油)和碳质炭。完成脱挥发分大约需要 2 毫秒到 20 毫秒。
- VM 的点火和燃烧主要产生 CO2(二氧化碳)和 H2O(水蒸气)。这需要几毫秒。
- 残余炭的部分燃烧由 O2 进行。炭燃烧贡献了燃烧过程中释放的大部分热量。与 VM 的燃烧不同,VM 向富氧气氛扩散(导致反应面积大),用于炭氧化的 O2 必须输送到相对较小的颗粒表面。因此,炭氧化是一个较慢的过程。只要 VM 被释放,由于 VM 的高化学计量要求,O2 就无法接触炭表面。
- 通过 CO2 和 H2O 将残余炭气化,生成 CO(一氧化碳)和 H2(氢气)。这是所有这些过程中最慢的反应,主要发生在滚道外。
煤的燃烧特性而不是焦炭的燃烧决定了滚道中的气体成分和温度分布,因为它们优先燃烧。图 1 说明了在滚道内发生的一些煤燃烧步骤,以及气体成分如何变化。大部分O2在风口附近消耗,而中间产生富CO2气氛,而在管道末端产生富CO气氛。
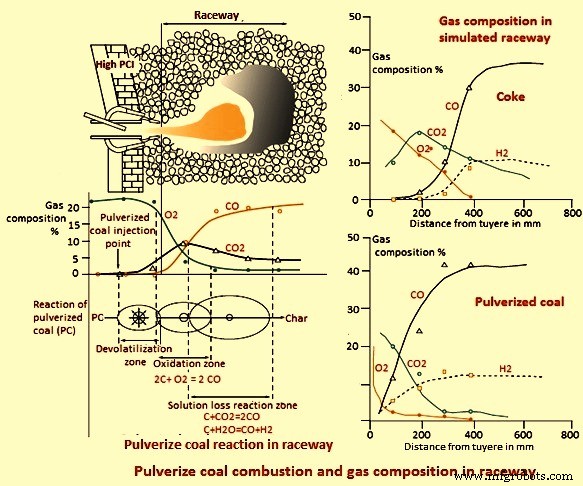
图1煤粉燃烧及滚道内气体成分
燃烧程度(燃烧效率),以及由此从滚道中输送出来的未燃烧材料的数量,取决于几个参数,包括 (i) 煤的特性,例如 VM 含量、粒度和密度,以及 (ii) ) 操作条件,例如,爆炸气体的成分和温度,以及喷枪的位置和设计。
图 2 中的曲线显示了燃烧效率和煤粉喷射率,这是基于使用材料和热平衡模型从 BF 中的碳平衡研究煤粉的最大喷射率时所做的研究。
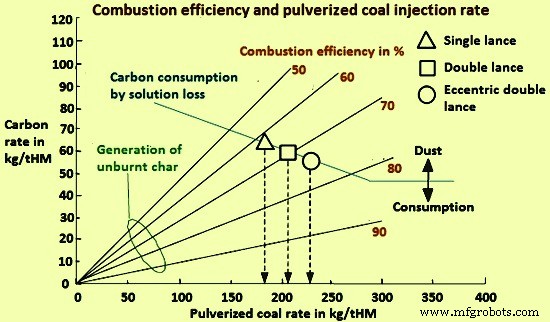
图2燃烧效率与喷煤量
根据开展的各项调查,总结出加强滚道燃煤的措施。
- 用 O2 丰富爆炸效果。然而,需要考虑爆炸氧气对燃烧程度的非线性影响。随着 O2 含量的增加,燃烧速率的增加变得更小。
- 在引入风口腔之前将煤粉与 O2 进行初步混合。
- 使用混合煤(通常是 VM 含量高或低的煤)和燃料混合物,以保持高燃烧度和高焦炭/煤替代率。
- 用氧化铁(细铁矿石和含铁废料等)、碳酸盐和其他富氧添加剂喷煤。
- 使用化学和物理现象,例如催化、极化和其他作用。
- 优化磨煤,具体取决于操作条件和煤炭特性。
煤阶的影响
煤粉在滚道中的燃烧和气化行为受其性质的影响。下面介绍这些性质对火焰温度(FT)和燃烧效率(CE)的影响。
PCI 对 FT 有冷却作用。 FT 是一个重要参数,因为它影响炉渣和金属化学、存在的碱金属元素的蒸发和再循环,以及金属在炉膛中的流动。 FT 很难测量,因此通常根据滚道区的能量平衡来计算。计算值称为“管道绝热火焰温度”(RAFT)或理论 FT。根据所做的假设,RAFT 计算可能因一个 BF 而异,因此可能无法直接比较值。每个 BF 都有一个最佳 RAFT,具体取决于炉料成分和渗透率、焦炭质量和吹炼速度等因素。喷煤会降低 RAFT(与全焦操作相比),因为它会促进吸热反应。低 VM 和高 VM 煤分别在 80 摄氏度至 120 摄氏度和 150 摄氏度至 220 摄氏度/100 kg/tHM 的范围内降低 FT。一般来说,煤中的H2/C(碳)比越高,冷却效果越好。 RAFT 也随着喷煤率的增加而减小。提高鼓风温度和/或富氧量,和/或降低鼓风水分可以补偿煤的冷却效果。
模拟高炉环境条件下的燃烧实验表明,CE 通常随着煤 VM 的增加而增加。与低挥发分 (LV) 和中挥发分 (MV) 煤相比,HV(高挥发分)煤很容易气化,产生更多气体,CV 更低,焦炭量更少。因此,对于低阶煤,气体燃烧比焦炭燃烧更重要。如果气体燃烧不完全,会形成烟灰,这会导致 BF 离开滚道时的渗透性变差。与未燃烧的炭相比,烟灰的反应性较低。
脱挥发分的程度受煤粒度的影响,粒度越细,脱挥发分越完全。随着煤 VM 含量的降低,最终 CE 由炭反应控制,因为 VM 的点火和燃烧速度很快。具有较高反应性的炭具有较高的 CE。人们经常争论说,在滚道中发生的高温下,化学反应变得不那么重要,因为燃烧速率受到 O2 扩散到颗粒的速率的限制,而燃尽时间更多地取决于颗粒大小和 O2 浓度。结合较短的停留时间,煤之间的炭反应性差异的影响在滚道中可能不是很显着。还有其他意见指出,鉴于所使用的小颗粒尺寸(PCI 中大于 80% 小于 75 微米)和滚道中存在的高度湍流条件,炭燃烧的总速率通常受固有的影响炭的化学反应性。炭反应性在滚道之外当然很重要。在上炉条件下,焦炭气化很可能受化学反应速率的控制。因此,炭的整体气化反应速率可能受炭对CO2的化学反应性的影响。
一般来说,焦炭的反应性随着煤 VM 的增加而增加,也就是说,HV 煤通常比 LV 煤产生更多的反应性焦炭,因此燃尽效果更好。也有例外,因为炭的反应性受许多因素的影响,包括 (i) 其形态(表面积和孔隙率),(ii) 其最终结构,(iii) 其组成,以及 (iv) 操作条件。炭的燃烧速率和反应性部分取决于颗粒的大小及其孔结构。孔隙结构控制活性气体向煤颗粒内部的供应,并为反应提供可变的内表面。
受其结构影响的炭碎裂会增加外表面积。在高加热速率下产生更高比例的具有薄壁空腔和更高大孔率和大孔表面积的炭颗粒。一般来说,这些类型的炭比那些具有较厚壁和较低孔隙率的炭更容易碎裂,因此具有更高的炭反应速率。碎片化可能是一些运营商发现 VM 对煤的可燃性影响不大的原因之一。在高温下由较高等级 (LV) 煤形成的炭通常更有序,因此反应性更低。随着温度的升高,高度各向异性的炭漂珠的发展也降低了炭的反应性。这些煤因此受益于较低的鼓风温度以提高可燃性。
煤的微量成分的变化可以解释燃烧反应性的差异,特别是在类似等级的煤之间。惰性物质通常被认为是“惰性的”(不反应的)。然而事情并没有这么简单。事实上,并非所有惰性物质都没有反应性,也并非所有镜质体都具有反应性。镜质体、惰性体,甚至是褐煤体,都可能导致碳质残渣中的未燃碳。还注意到,虽然富惰性煤焦在 500 摄氏度时的反应性基本上低于富镜质体煤焦,但这在高温(1300 摄氏度)下不再重要。在滚道内非常强烈的燃烧条件下,煤的可燃性差异可能会大大减小。
煤的燃烧性能可因组成矿物的催化作用而改善或因矿物浓度过高而延缓。 SiO2 和 Al2O3(氧化铝)可以减慢反应速度,而钙(Ca)、镁(Mg)、铁(Fe)和碱类可以改善反应速度,在低阶煤中催化效果更明显。然而,富矿物颗粒的可燃性提高,不是由于催化作用,而是由于反应气体通过矿物和微晶矿物界面的有利扩散。炭反应性和单个无机相之间缺乏明确的相关性可能与温度对煤矿物转化的影响不同有关。虽然通常首选反应性高的煤和木炭,但反应性过高会导致炉况不稳定。
混合可以稀释煤的不利燃烧特性。但混合煤的燃烧性能比单一煤的燃烧性能要复杂得多。每种煤组分在不同的温度和不同的时间脱挥发分和燃烧,因此它们的燃尽可能有很大差异。此外,混合物中各种煤之间可能发生相互作用,这使混合物燃烧行为的预测变得复杂。相互作用首先发生在粉碎机中,其中组分煤的尺寸分布可能存在很大差异,尤其是在每种煤的硬度存在显着差异的情况下。歧化也会发生,影响所得颗粒的矿物和岩相组成,以及随后的燃烧行为。
组分煤之间的相互作用可以增加混合物的可燃性。例如,LV 煤的可燃性可以通过与 HV 煤混合来提高。 HV煤释放更多VM有助于形成更高的气体温度场,然后加热LV煤。这促进了它的脱挥发分、点火和燃烧。 HV煤比例越高,协同效应越明显,达到一定比例。在模拟高炉条件下,含有约 70 % HV 煤(含 32.5 % VM)和 30 % LV 煤(含 20 % VM)的混合物燃尽率最高。
粒度效应
煤的燃烧性能受其粒度的影响。 For complete conversion, and hence effective utilization of the injected coal, the heating up, devolatilization, pyrolysis and combustion of the particles need to take place in the period between their entry into the hot blast and the raceway boundary. Normally, greater amount of VM is released with reducing coal particle size. This can facilitate gas phase combustion.
Finer particles have higher specific surface areas and thus higher heating rates. The granular coals releases lower amounts of VM than when they are pulverized. Calculated pyrolysis yields indicate that nearly all the VM from the pulverized coals are released whereas it is incomplete in case of the granular coals. The presence of residual VM in the granular coals affects the subsequent CO2 gasification reactivity of the chars. It has also been shown that the extent of devolatilization in the finer particles (45 microns to 75 microns) is more complete than the larger particles (75 microns to 150 microns). The effect is more pronounced for the LV coal (15 % VM) compared to the HV coal (37 % VM). This is since a higher VM release can result in more soot and tar production, produced from secondary reactions of the volatiles. The reactivity of the soot is lower than that of the unburned char. Thus, the lower is the soot formation; the better is the BF stability.
The CE (or burnout) of coal normally increases with decreasing particle size since a higher surface area is available for reaction. Larger particles require a longer time for burnout. The increase is more pronounced as VM content increases in coals. However, the particle size effect is also dependent on O2 stoichiometry, as well as coal rank (and char reactivity). It has been found that larger particles of coal generally have a higher CE (degree of burnout) at O2/C ratios of greater than 2 (fuel lean conditions) under simulated BF conditions. The smaller particles have higher CE under fuel rich conditions (O2/C ratio less than 2).
Operational factors
The effective use of coal needs operational changes to compensate for alterations in the raceway parameters and their effect elsewhere in the BF (such as the thermal state, slag regime and gas dynamics). Measures to intensify the combustion of coal in the tuyere/raceway region, and hence increase the injection rate include (i) increase the amount of O2 in the tuyeres, and (ii) adjustment in the blast temperature and moisture. There are some other measures taken to improve coal combustion, such as preheating the coal and the use of additives. Further, the choice of particle size, and hence the grinding parameters, can also influence the CE.
Oxygen can be added to the tuyere by (i) enrichment of the hot air blast, (ii) injection through the coal lances, and (iii) separate O2 lances. The addition of O2 results into higher availability of O2 for the participation in the combustion of coal in the raceway. Hence, the CE of the coal increases. However, the influence of O2 enrichment on CE is limited. It has been shown through calculation that the CE increases by around 6.7 % for a HV coal (34.5 % VM) and 3.3 % for a LV coal (14 % VM) when O2 enrichment of the hot air blast is raised from 0 % to 6 % by volume. With higher O2 enrichment, CE can actually decrease due to insufficient mixing. Increasing O2 enrichment increases the diffusion of O2, but diminishes the volume of combustion gas which transfers heat to the coal particles. Thus, there is the non-linear effect of blast O2 content on the degree of coal combustion.
Oxygen enrichment of the hot air blast produces both a reduction in bosh gas flow and a rise in FT. The former effect can help counteract the increase in the burden resistance (lower permeability) and the pressure drop associated with high injection rates. The latter effect can help compensate for the cooling effect of the decomposition of the coal VM. The CO and H2 contents also increase with O2 enrichment, resulting in the improved reduction of the iron ore in the central shaft. The CV of the top BF gas normally improves with O2 enrichment.
The lower limit of O2 enrichment is generally determined by the amount needed to maintain the required RAFT, with more O2 needed as the VM content of the coal increases. If the FT becomes too high, then burden descent can become erratic. Too low a FT hampers coal combustion and melting of the ore burden. The upper limit is dependent on maintaining a sufficient top gas temperature. As O2 is increased, the gas mass flow within the BF decreases, which decreases the heat flow to the upper region of the furnace for drying of the burden. The upper limit of the top gas temperature is also governed by the need to protect the top gas equipment. Other limitations to O2 enrichment include its cost and availability.
The position and design of the injection lance influence the CE and ash deposition in the tuyere. However, oxy-coal lance injection (co-annular injection) can produce an insulating effect around the coal particles, resulting in less coal combustion inside the tuyere. This effect carries over into the raceway, and less combustion is the end result. Lowering the O2 lance injection rate in this case improves the CE.
The key measure for combustion at high injection rate is a high blast temperature. O2 enrichment plays a more important role as a means of controlling gas flow in the BF rather than controlling the coal combustion. Normally, a higher hot blast temperature is a cost effective measure than O2 enrichment since it allows a lower consumption of O2. Increased blast temperature also reduces coke consumption, typically 10 kg/tHM for every increase of 40 deg C with PCI, and lead to a small rise in the raceway depth. A higher blast temperature is normally required as the VM of the coal increases. This is due to the lower char reactivity of the low VM coal.
Lowering of blast moisture can help to compensate for the cooling effects of PCI. If the RAFT becomes excessive, then blast moisture can be increased. Raising the hot blast moisture means more H2 in the bosh gas for iron ore reduction. The optimum RAFT in BF operating with higher H2 content can be lower than the BF operating with lower H2. Also, the blast velocity can be adjusted to not only improve coal combustion, but to maintain the needed length of the raceway zone which is critical for obtaining good conditions in the hearth.
Unburnt char
As the injection rate increases, the combustibility of coal tends to decrease resulting in unburnt material (such as char, fines, and fly ash) leaving the raceway. Some of these materials, along with coke debris, collect at the back of the raceway, in the bird’s nest, obstructing the rising gas flow and entrained solids in this area. The majority are swept upwards where they can accumulate under the cohesive zone, decreasing permeability and hence the productivity of the BF.
Changes in the permeability of the lower furnace zone can further affect the HM quality and slag viscosity. The unburnt material tends to collect at positions where large changes in the gas flow occur. Eventually it is entrained into the gas flow, passing through the cohesive zone coke slits, and up the shaft, where it can influence burden permeability, and is finally emitted with the top BF gas. Higher coal injection rates also increase the volume of combustion gases, and hence the gas flow, and change the heat load in the lower part of the BF. In addition, more slag is produced.
The deposition of unburnt fine material is a complex phenomenon consisting of several generation mechanisms, reactions, multiphase flow, buildup and re-entrainment. Different gas flow models have been developed to understand and predict the behaviour of fine material within the BF. With suitable burden charging patterns (such as central coke charging) and the use of stronger coke many of the problems relating to gas flow can be overcome.
Operating experience has shown that most of the unburnt material (char) is consumed within the furnace by the three mechanisms which are (i) gasification with CO2 and H2O, (ii) reaction with liquid iron (carburization), and (iii) reaction with slag. It is advantageous if the unburnt char participates in the ore reduction reactions, thus replaces more of the coke and lowers the amount of unburnt solids in the top BF gas. The three char consumption mechanisms are described below.
The gasification reaction of char with CO2 and H2O begins in the raceway, but because the residence time for fine particles is too short for appreciable reaction, gasification mainly occurs in the BF shaft. The reactions of char C with CO2 (the solution loss or Boudouard reaction) and H2O are slower than char combustion. The char obtained from coal competes with that from coke for CO2 and H2O. Char from coal is more reactive than the char from coke and hence is preferentially gasified. Therefore coke degradation by the solution loss reaction decreases with increasing PCI rates. In general, high VM coal char has a higher CO2 reactivity than low VM coal char. However the char reactivity in case of low VM coal can be improved by blending it with the high VM coal. The CO2 reactivity of coal blends is non-additive.
The reactivity of C in the unburnt char to CO2 and H2O is dependent not only on its surface area (particle size) but also on its structure and composition, as well as the operating conditions in the BF. It has been shown that the CO2 gasification reactivity of coal char increases with temperature upto 1500 deg C, especially between 1300 deg C and 1500 deg C. Complete char gasification usually requires a contact time of around 10 seconds at 1500 deg C. Since the residence time for particles at such high temperatures is too short in a BF, hence char gasification mainly occurs at decreasing temperatures in the furnace shaft.
The properties of char change as it moves up the BF, and thus its reactivity to CO2 and H2O. The reacting atmosphere is not uniform. As an example, the concentrations of CO, CO2, H2 and H2O vary at different locations within the BF. Injection of coal increases the bosh gas H2 concentration. Since the chemical reaction rate of H2 reduction is higher than that of CO, the extent of solution loss reaction diminishes as the bosh gas H2 rises. CO2 and H2O are present in the upper part of the BF due to the reduction of iron ore. Under the conditions here, char gasification by CO2 is expected to be controlled by the rate of the chemical reactions. In the lower part of the BF, char gasification is partly diffusion controlled. Hence, the overall reaction rate of char gasification is probably influenced by the chemical reactivity of char to CO2 in this region. Char reactivity towards CO2 is also influenced by its chemical structure, with less ordered structures being more reactive.
The presence of certain minerals in the char ash, such as Fe and alkalis, can catalyze the CO2 gasification reaction, whereas other minerals, such as SiO2 and Al2O3, can slow down the reaction. These catalytic effects become more prominent for low rank coals. Depending on its composition, ash can also retard the C conversion due to blockage of char particles as a result of increased proportion slag formation in the char particle. In the lower part of the BF, condensed alkalis from the recirculating gases (derived from coal, coke and iron ore) can have a catalytic effect. The loss of C by gasification increases the char ash content.
Carburization of the HM begins in the solid phase within the cohesive zone of the BF, and continues during descent of the metal droplets through the active coke, deadman and hearth zones. Unburnt char and fine material leaving the raceway can contact the dripping liquid metal in the bosh and hearth zones. C and other elements, such as Fe, Si (silicon) and S (sulphur), dissolve from the char into the liquid iron and hence influence the composition of the HM. The dissolution of C contributes to the carburization of liquid iron, and controls the level of char consumption by the HM. It becomes critical when the CE is low. If the HM is close to saturation when it reaches deadman and hearth, the unburnt material cannot be consumed, thus reduces the permeability in these regions. The C can come from unburnt coal as well as from coke. Since the dissolution rate of C from coal char is a slower process than that from coke, C from coke is preferentially consumed.
Carbon dissolution from unburnt char into liquid metal is influenced by the furnace operating conditions and the following factors.
- Char particle size – Unburnt char which maintains its pulverized form reacts very little with the liquid metal and the slag since it cannot penetrate into the liquid. If, however, the char particles are agglomerated into larger particles or captured by the larger pieces of coke, then they behave like bosh coke and carburize the liquid metal up to saturation. However, a tuyere probe sample taken at a BF in Australia indicated that ultrafine coal char particles can react with the dripping liquid metal, and that they are more readily dissolved than ultrafine coke particles. Experiments, though, have shown that the dissolution rate of C from coal char, though at larger particle sizes, is a slower process than that from coke.
- Char structure – Normally, the rate of dissolution improves as the C structure becomes more ordered.
- Char mineral matter – In general, SiO2, Al2O3 and MgO (magnesia) slow the C dissolution kinetics, whilst CaF2 (calcium fluoride) and Fe oxides increase the rate. The effect of CaO (calcium oxide) is less clear. The reaction of Ca (calcium) with S in the metal produces a layer of CaS (calcium sulphide) which can inhibit C transfer. The AFT is also one of the controlling mechanisms which limit the C dissolution. The formation of an ash layer on the carbonaceous material reduces the surface area available for dissolution, thus retarding C dissolution rates. Low AFT allows easy removal of the ash, in the form of liquid slag. This results in constant exposure of fresh C surface to the liquid iron, permitting the mass transfer of C to the liquid iron.
- Liquid iron composition – It changes over time. The C dissolution rate is typically decreased as the C content of the liquid iron increases. Higher S content also retards the C dissolution. Combustion of coal and coke releases sulphur oxides (SOx) which can react with the descending metal and slag.
Unburnt char, ash, fines and coke can interact with the dripping slag. The slag composition changes as it moves down the BF, with the Fe oxide concentration being continuously reduced. The reactions at the interface between the solid char and liquid slag play a major role in char consumption since they influence the kinetics of the reduction reactions and the contact area between the slag and char available for reaction.
Factors influencing unburnt char interactions with the slag include the slag composition, char C content, and char ash content and composition, as well as the furnace operating conditions. Char consumption by slags basically occurs through the following mechanisms.
- Reduction of the Fe oxides in slags by C in the char – The wetting characteristic has a significant effect on the dominant reduction mechanism taking place. The wetting characteristic of slags varies with slag composition, temperature, time, and carbonaceous material. Wetting varies as a function of time since the reduction of Fe oxide in the slag by char, and the dissolution of the char ash components into the slag, results in continuous variations in the slag and char compositions. An increase in temperature normally results in improved wettability at the slag/C interface. Reduction rate usually increases with increasing slag FeO (2 % to 10 %) content and with increasing reaction temperature (1300 deg C to 1600 deg C). In general, coal chars are poorly wetted by slag containing more than 10 % FeO at 1400 deg C and 1500 deg C. A faster reaction rate for coke suggests that coke fine is preferentially consumed before coal char.
- Reduction of SiO2 in slag by C of the char – It is a function of temperature. At temperatures less than 1500 deg C, only reduction of FeO takes place. At higher temperatures, both SiO2 and FeO in the slag are reduced, thus resulting in increased consumption of the char. SiO2 is reduced by C, through gaseous SiO, to Si or silicon carbide (SiC). Self-reduction of SiO2 in the char ash by C can also occur, resulting in further consumption of the char. The reduction kinetic of SiO2 is influenced by the wettability of char by the slag. Wetting behaviour is improved with an increase in slag SiO2 content, and with an increase in temperature (1500 deg C to 1700 deg C). Greater amounts of SiO2 and FeO in the char ash facilitate the slag/C interaction, leading to improved consumption of these oxides through reduction reactions.
- Interaction between components in the slag and char – This leads to the assimilation of char ash components such as S. In addition, the reduction of MgO in slag by char C can lead to further consumption. Self-reduction of the oxides in the char ash by C can also contribute to char consumption.
Slag viscosity has also a role to play. The presence of unburnt char in the slag can interfere with tapping by increasing slag viscosity, whereas absorption of char usually increases the fluidity of the bosh slag. Changes in slag mobility can affect the position and shape of the fluid and cohesive zones. A high viscosity slag around the tuyeres leads to serious gas flow problems. Slag viscosity is a complex function of slag composition, temperature and partial pressure of O2. Also unburnt char, coke, and unburnt ash from the coal can interact with the slag. All of these carbonaceous materials contribute oxides to the slag. In general, higher amounts of SiO2 or Al2O3 (acidic components) increase slag viscosity, whereas a higher basicity (higher CaO or MgO) lowers slag viscosity because of de-polymerization of the silicate network. Slag viscosity decreases with increasing FeO (0 % to 20 %) content at a fixed basicity. Basicity is normally determined by the CaO/SiO2 ratio. Since the slag does not completely absorb the char and ash in the bosh region, bosh slag usually has a higher basicity than tapped slag. The addition of fluxes can help in solving slag formation problems.
制造工艺


