高炉炼铁化学
高炉炼铁化学
以低焦比运行的现代高炉 (BF) 是一种高效的处理装置,主要是因为逆流气固反应器的固有特性。成功使用这一概念需要装入熔炉的每种材料具有统一的物理特性,并具有统一的成分。此外,每种材料在通过熔炉向下移动到发生熔化的地方时都应保持这种良好的物理特性。
随着氧化铁、焦炭和炉渣形成材料向下移动通过熔炉的烟囱,会发生几个重要的交换过程。热量从主要由一氧化碳 (CO)、二氧化碳 (CO2) 和氮气 (N2) 组成的上升炉气中带走,并转移到下降的炉料中。氧气 (O2) 从下降的氧化铁中去除并转移到上升的还原气体中。因此,在这个非常有效的逆流反应器中,会发生化学反应,随着装料的下降,装载材料的温度升高,还原铁、氧化铁和造渣材料开始熔化,最后液态金属和炉渣聚集在炉膛。加入炉内的大部分焦炭在风口处与热空气中的氧气一起燃烧,以提供热量和还原剂 CO。
当装入高炉顶部的炉料和焦炭通过烟囱下降时,它们被从风口上升的热气预热。由于这种预热,当焦炭到达靠近风口的炉子下部并与热风空气接触时,焦炭会以极大的强度燃烧。然而,由于温度非常高(约 1,650 摄氏度)和大量碳 (C) 以焦炭形式存在,形成的 CO2 不稳定,会立即与额外的碳反应形成 CO。因此,燃烧BF中的碳(焦炭)可以用化学方程式2C + O2 =2CO表示; ΔH =+110,458 kJ/kmol。在现代高炉的运行中,每生产一吨铁水,就有 250 公斤到 400 公斤的碳以这种方式发生反应。该反应是冶炼操作的主要热源,还会产生还原气体 (CO),该气体会上升到炉膛,在此预热并还原炉料中的大部分氧化铁。
鼓风空气中的任何水分 (H2O) 也会与燃烧区焦炭中的一些碳发生反应。这种反应不像燃烧那样产生热量,而是消耗热量。然而,对于每单位的碳,该反应产生的还原气体比碳在空气中燃烧时产生的还原气体多。当碳在空气中燃烧时,它只产生一个单位的 CO,但当它与 H2O 反应时,它会产生一个单位的 CO 和一个单位的氢气 (H2)。因此,在某些情况下,如果装载材料的固有还原率低于正常水平,并且可以获得相对较高的热风温度(在 1,000 摄氏度和 1,200 摄氏度之间),则认为保持通过添加水分(蒸汽)以增加高炉气体的还原能力,使鼓风的水分含量保持在一致的高水平。辅助燃油喷射提供了类似的优势。这种化学反应用方程式 C + H2O =CO + H2 表示; ΔH =+131,378 kJ/kmol。另一个好处来自于在炉内还原气体中引入(或增加)氢气。随着氢气百分比的增加,气体的密度降低。这导致等量的还原气体减少了对负担的抵抗。
在温度低于 925 摄氏度的高炉上部,上升的气体开始还原炉料的氧化铁。在此温度下,化学平衡阻止所有 CO 和 H2 用于还原(平衡 CO / CO2 比率约为 2.3 用于方晶石的还原,如果该比率低于此值,铁将被重新氧化。因此,CO 或 H2 与氧化铁的分子比率约为化学计量反应所示量的三倍(i ) 1/2 Fe2O3 + 3/2 CO =Fe + 3/2 CO2;ΔH =+12,866 kJ/kmol,(ii) 1/3 Fe3O4 + 4/3 CO =Fe + 4/3 CO2;ΔH =+3940 kJ/kmol,(iii) FeO + CO =Fe + CO2;ΔH =–16,108 kJ/kmol,(iv) 1/2 Fe2O3 + 3/2 H2 =Fe + 3/2 H2O;ΔH =+ 48,953 kJ/kmol,(v) 1/3 Fe3O4 + 4/3 H2 =Fe + 4/3 H2O;δ H =+51,042 kJ/kmol,和 (vi) FeO + H2 =Fe + H2O;δ H =+ 25,104 kJ/kmol。
过去,这种类型的还原称为间接还原,而在较高温度下发生的类型称为直接还原。然而,这种命名法已经变得令人困惑,因为这些相同的化学反应在描述 DRI 过程(如 Wiberg、HIB、FIOR 和类似过程)时被称为直接还原。因此,这些术语通常不像过去那样使用。
在温度较低的炉子上部没有被还原的氧化铁部分要在温度非常高的下部被还原。由于 CO2 和 H2O 在存在大量焦炭的情况下在这些温度下不稳定,因此它们与碳的反应几乎与它们形成时一样快。因此,炉子这部分的整体还原反应可以表示为反应FeO+C=Fe+CO;无论反应物是 H2 还是 CO,delta H =+156,482 kJ/kmol。该反应是通过代数添加两个反应 FeO + CO =Fe + CO2 得到的; delta H =–16,108 kJ/kmol,CO2 + C =2CO; delta H =+172,590 kJ/kmol 或反应 FeO + H2 =Fe + H2O; delta H =+25,104 kJ/kmol,H2O + C =CO + H2; delta H =+131,378 kJ/kmol。
还原反应FeO+C=Fe+CO吸收大量的热量,因此,以这种方式发生的还原量越大,向炉子提供的热量就越大。该反应还产生 CO,这是用于 BF 烟囱中发生的还原反应的气体。在大多数情况下,当大约三分之一的还原根据反应 FeO + C =Fe + CO 完成,其余根据反应 Fe2O3 + 3 CO =2 Fe + 3 CO2 通过FeO + H2 =Fe + H2O。
该过程的热量并不完全由焦炭的燃烧产生,因为在大多数高炉中,大约 40% 的热量来自热风空气的显热。相当一部分燃料可以通过风口以天然气、焦油、燃料油或粉状或颗粒状煤的形式经济地喷射。在这种情况下,燃料中的碳燃烧成 CO,但由于存在大量焦炭,氢气仍以 H2 形式存在,直到在风口上方某处还原氧化铁时才会被氧化。
炉料中的含铁成分是铁、Fe2O3 和 Fe3O4 的简单氧化物。天然矿石通常是赤铁矿(Fe2O3)或磁铁矿(Fe3O4)。颗粒主要是Fe2O3。铁矿石烧结矿的成分范围从 Fe2O3 和 Fe3O4 到包含磁铁矿、铁橄榄石、2FeO.SiO2 和铁酸二钙的熔融混合物。氧化铁的还原通常分步进行。与 CO 的反应由方程式 (i) 3Fe2O3 (s) + CO (g) =2Fe3O4 (s) + CO2 (g) 给出; ΔH -48 kJ,(ii) Fe3O4 (s) + CO (g) =3FeO (s) + CO2 (g); delta H -21.7 kJ,和 (iii) FeO (s) + CO (g) =Fe (s) + CO2 (g); ΔH -11 kJ。这些反应是在连续更高的温度下完成的,并且在炉子的下方更远。
通过上升的气体完成这些反应需要连续更高百分比的 CO。应该认识到,对于每个反应,气体中的全部 CO 都转化为 CO2 是不可能的。例如,三个方程的常数 K3 和 K3 =P CO2 / P CO 给出了一个平衡比,这取决于温度。在 800 摄氏度时,平衡气体混合物包含约 65% 的 CO 和 35% 的 CO2。如果在此温度下与 FeO 和固体铁接触的气体中的 CO2 含量超过此值,则存在的铁往往会被氧化回 FeO。因此,为了迫使这些反应发生,如图 1 所示,在每个步骤中,气体中都需要有相当浓度的 CO,并且不可能通过反应将 CO 完全转化为 CO2。图 1 显示了 Fe-C-O 和 Fe-H-O 系统的稳定性图。 Fe-C-O体系中的S曲线代表“溶液损失”或Boudouard或反应。
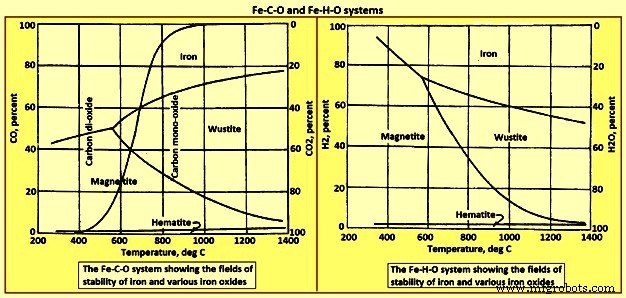
图 1 Fe-C-O 和 Fe-H-O 体系
由于辅助燃料中的氢气以及燃料和空气中的水分,离开风口的气体也可能含有高达 2% 或 3% 的氢气。可以将蒸汽添加到热空气鼓风中,以帮助控制熔炉。焦炭和燃料中的碳对蒸汽的还原通过整个反应 H2O (g) + C (s) =CO (g) + H2 (g) 进行; ΔH =131.3 kJ。该反应是吸热反应,而爆炸中的氧将碳氧化形成 CO,方程式为 C (s) + 1/2 O (g) =CO (g); delta H =-110.5 kJ 是放热的。用氢还原氧化铁也是通过步骤(i)3Fe2O3(s)+H2(g)=2Fe3O4(s)+H2O(g)进行的; ΔH =-7.1 kJ,(ii) Fe3O4 (s) + H2 (g) =3FeO (s) + H2O (g); delta H 62.9 kJ,和 (iii) FeO(s) + H2 (g) =Fe (s) + H2O (g); delta H =30.2kJ。温度影响这些反应的平衡。
水煤气变换反应CO2(g)+H2(g)=H2O(g)+CO(g); delta H =41.2 kJ 可以在气相中的各种物质之间发生,以重新分配氧气并使含氢和含碳气体物质达到平衡。该反应需要很少的热量,平衡常数 (P H2O.P CO) / (P H2.P CO2) 在 825 摄氏度时为一。烟囱中的气体与焦炭中的碳以及氧化物发生反应电荷中的铁。 CO 和 CO2 与碳作为石墨的整体反应是“溶液损失”或 Boudouard 反应 CO2 (g) + C (s) =2CO (g); ΔH 172.4 kJ。在高于 750 摄氏度的温度下,反应平衡强烈向右移动。低于 600 摄氏度,平衡强烈向左移动,导致碳在炉料中以烟灰形式沉积 2CO (g) =C (s ) + 二氧化碳 (g);增量 H =-172.4。从图 1 的左下角到顶部中心的“S”形曲线代表平衡。温度和成分高于线的气体倾向于通过第二个反应沉积碳,而其成分和温度低于线的气体会根据第一个反应氧化碳。
高温下碳溶液反应的主要影响是在需要的地方产生的热量相对减少,并且在高于 700 摄氏度的炉子区域的气体中的 CO 浓度增加。后一种条件是特别理想,因为它增加了气体的体积并有助于热传递。需要说明的是溶失反应与反应FeO(s)+CO(g)=Fe(s)+CO2(g)的结合; delta H -11 kJ 对应于方程式 FeO (s) + C (s) =Fe (s) + CO (g) 给出的碳对 FeO 的“直接”还原; ΔH =131.3 kJ。从图 1 可以明显看出,通过烟囱的气体通常不能与焦炭中的碳和下降炉料中的氧化铁平衡。高炉烟囱内气体成分与温度的实际关系在很大程度上取决于所采用的实际做法。
氧化物的相对稳定性
在 Ellingham 图(图 2)中绘制了各种氧化物的相对稳定性与温度的关系。 Ellingham 图对于理解高炉中氧化物的行为非常有用。相对稳定性是根据氧化物形成的自由能来测量的。氧化物形成的负自由能越高,氧化物稳定性越高。这意味着位于图表上部的氧化物具有相对较低的稳定性,而位于图表下部的氧化物具有较高的稳定性。位于图中心的氧化物具有中等稳定性。稳定性相对较低的氧化物包括氧化钾、氧化钠、氧化磷和氧化铁。具有中等稳定性的氧化物包括氧化锰、氧化铬、二氧化硅和氧化钛。 .稳定性高的氧化物有氧化铝、氧化镁和石灰。
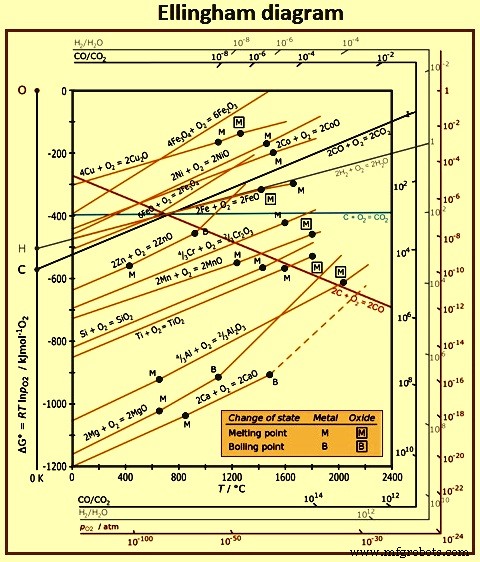
图 2 Ellingham 图
根据元素对氧的亲和力来考虑该图也很有用。例如,位于图表顶部的元素对氧的亲和力较低,而位于图表底部的元素对氧的亲和力很高。这意味着顶部的氧化物相对容易还原,而底部的氧化物则难以还原。这由氧化磷形成线显示,该线位于氧化铁形成线上方,温度对应于高炉炉膛中的温度。这意味着氧化磷的稳定性低于氧化铁,因此,由于炉中的还原条件足以还原氧化铁,因此基本上所有进入炉内的磷最终都会进入铁水。另一方面,稳定的氧化物如氧化铝、氧化镁和石灰在高炉条件下不会被还原,最终进入渣相。氧化锰、氧化铬、氧化硅、氧化钛等稳定性中等的氧化物被部分还原,产生一些溶解在铁水中的锰、铬、硅和钛,而其余未还原的氧化物则构成炉渣的一部分。
Ellingham 图是建立在单位活度的纯元素与一摩尔氧气反应形成单位活度的纯氧化物的基础上构建的。热力学术语“活度”是一个特别有用的概念,用于讨论溶解在液态铁中的元素或溶解在液态渣中的氧化物的行为。例如,当少量元素(如氧或硫)溶解在钢液中时,它们的活度通常可以被视为等于它们的浓度百分比。但是,在其他元素浓度较高的情况下,例如铁水中的碳,硫的活度高于浓度,而氧的活度低于浓度。在这种情况下,区分活动和注意力很重要。 · 溶液中组分的浓度是存在多少组分的量度。 · 组件在解决方案中的活动是衡量组件实际行为方式的指标。
Ellingham 图上除涉及碳的线外,所有线都具有正斜率,表明氧化物稳定性随温度升高而降低。氧化钾、氧化钠、氧化镁和石灰的氧化物的线各自显示出在对应于相应金属沸点的温度下的斜率增加。由碳和氧生成 CO2 的线斜率几乎为零,表明稳定性随温度升高变化不大,而 CO 的斜率很大,这意味着 CO 的稳定性实际上随着温度升高而增加。两种碳氧化物的谱线在 700 ℃左右交叉。高于此温度,CO 比 CO2 更稳定,而在较低温度下,CO2 比 CO 更稳定。
碳-氧反应
在大约 1,000 摄氏度至 1,200 摄氏度的温度和 0.2 兆帕至 0.3 兆帕的压力下,通过风口注入的预热空气在每个风口前产生一个梨形反应区。该区域的温度约为 2,000 摄氏度,过量氧气和焦炭首先发生快速反应,生成 CO2。这是一个放热反应(C + O2 =CO2)。在该区域之外,不再有可用的游离氧,CO2 与过量的焦炭反应生成 CO(CO2 + C =2CO)。这被称为 Boudouard 反应并且是吸热的。将这两个反应结合起来,产生了碳与氧气部分燃烧以提供 CO 的反应。(2C + O2 =2CO)。形成一摩尔 CO2 时放出的热量大约是形成一摩尔 CO2 的三倍半,BF 效率的一种衡量标准是焦炭中的碳转化为 CO2 的程度。
低于 700 ℃,CO2 比 CO 更稳定,第二个反应向左进行(2CO =C + CO2)。该反应通常被称为碳沉积。 700℃以上,CO比CO2更稳定,第二个反应向右进行。这种反应有时被称为碳溶液损失反应,在这个意义上意味着一种消极的行为。另一方面,该反应代表了在 700 摄氏度以上的炉内区域内还原气体的再生。这是焦炭在高炉内的重要功能之一,并且特别理想,因为它增加了气体的体积并有助于加热转移。然而,这种反应是吸热反应,当它发生在风口区内时,它会在高温很重要的位置产生冷却效果。
图 3 显示了温度对焦炭和含有 CO 和 CO2 在 0.1 MPa 和 0.3 MPa 压力下的平衡反应的影响,这在现代高炉实践中更为典型。在图的右侧,CO比 CO2 更稳定,而在较低温度下,在图的左侧,CO2 比 CO 更稳定。从该图可以清楚地看出,在 1,000 摄氏度以上,CO2 与焦炭平衡的百分比基本上为零。另一方面,在低于 400 摄氏度的温度下,CO 的浓度很小。因此,随着温度在1000℃到400℃之间降低,CO的稳定性降低,而CO2的稳定性增加,两种气体与焦炭平衡的分压相当大。
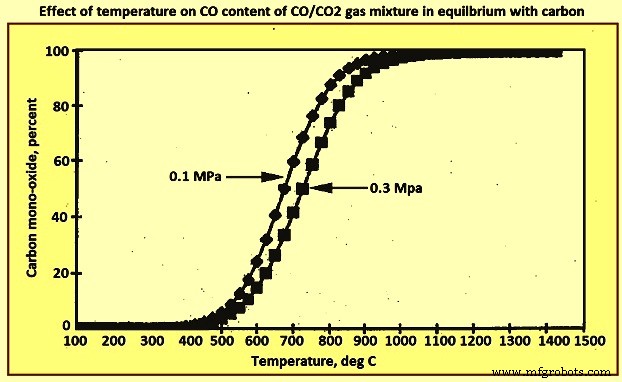
图3温度对与碳平衡的CO/CO2气体混合物中CO含量的影响
离开炉顶的气体通常在 200 摄氏度左右,如果与焦炭达到平衡,则 CO 与 CO2 的比率约为 10 次方 -5。事实上,该比率通常在 1 和 3 之间,即气体的还原性比从平衡考虑中预测的要大得多,并且没有充分利用气体的还原潜力。这意味着焦炭率超过了理论要求。气体和焦炭之间缺乏平衡可主要归因于烟囱中的高气体速度。气体在炉内的停留时间仅为 10 秒左右,并且会出现极高的速度,特别是在松散堆积、富含焦炭的地区。另一个因素是气体温度在炉内上升时会下降约 1800 摄氏度,因此几乎没有机会保持平衡。
积碳反应
由于在低于 1,000 摄氏度的温度下,高炉烟囱内气体的 CO 含量远高于所需的含量,因此存在进行碳沉积或烟灰反应的驱动力。这种驱动力在 500 摄氏度和 700 摄氏度之间特别强。温度和成分高于图 3 线的气体倾向于通过反应 2CO =C + CO2 沉积碳,而成分和温度低于线根据反应 CO2 + C =2CO 氧化碳。幸好积碳反应迟缓,永远无法达到平衡,否则会严重堵塞堆顶料仓内的空间。
这又会导致还原气体的不规则流动和负载的不均匀下降。即使对于部分反应,也需要合适的催化表面,碳可以在其上成核和生长。铁颗粒、部分还原的铁矿石和碳化铁都被建议作为可能的催化剂。氢气和水蒸气似乎可以增强反应,而氮和硫化合物,例如氨、硫化氢和二硫化碳作为抑制剂。氧化锌和碱性化合物与硫的抑制作用相反,虽然这些化合物在炉子中的浓度通常很小,但它们在炉膛中的高温下会挥发并在烟囱的较冷区域再次冷凝。累积效应是此类化合物可以抵消硫的影响。由反应沉积的碳呈非常细碎的形式,有些可以容纳在铁矿石颗粒的孔隙中,并再次向下堆放。这会以多种方式影响还原过程。
由于碳的活性及其与矿石的紧密结合,固体碳的还原可以在比焦炭还原所需的温度更低的温度下进行,特别是因为焦炭不能渗透孔隙并且还原只能在点处进行固体颗粒之间的接触。这种还原的速率取决于氧从颗粒内部扩散到接触点的速率。在炉的上部,与气态还原相比,焦炭的还原可以忽略不计。只有在 1,000 摄氏度左右,当气态反应受到熔渣形成的阻碍时,它才会变得相当重要。相比之下,在低至 800 ℃ 的温度下,沉淀碳的还原作用就可以发生。
孔内反应过程中CO的形成往往会在颗粒内打开较深的裂缝,从而增加气固接触面积,并提高气态还原的效率。当气体还原反应在颗粒的孔隙内产生CO2时,它可以通过与孔隙中的碳反应迅速再生为CO,从而使反应继续进行。
不幸的是,积碳反应也会产生一定的不利影响。该反应会导致耐火材料在温度约为 500 摄氏度至 550 摄氏度的区域中沉积在活性铁斑上,例如在堆垛较低层的外壳中,或在上部的内壳中水平。如果积碳过多,会使矿球或烧结矿碎成粉末,造成气流不规则,炉料下降不均匀。
由于碳沉积反应是放热的,因此出口气体的温度升高。尽管碳沉积反应的整体效果值得商榷,但某些事实仍然存在。该反应确实降低了出口气体的 CO/CO2 比率。该反应使一定量的碳再循环,否则这些碳将被排出炉外,从而增加了与碳反应的时间,提高了还原过程的化学效率。
氧化铁的还原
氧化铁被 CO 还原的反应可以表示为 (i) 3Fe2O3 + CO =2Fe3O4 + CO2,(ii) Fe3O4 + CO =3FeO + CO2,和 (iii) FeO + CO =Fe + CO2。这些反应是在越来越高的温度下完成的,如图 1 所示,CO 的百分比越来越高。这意味着相对容易实现的反应 (i) 和 (ii) 可以在上部区域内发生。炉。需要从铁中去除最后一部分氧气的反应 (iii) 实际上是最难实现的,因此在温度较高且还原气体的 CO 含量较高的炉子下方发生。 570℃以下,非化学计量方维氏体相(FexO)不稳定,有可能将磁铁矿直接还原成铁。
在任何特定温度下,还原特定氧化物所需的气体混合物中的 CO 含量最低。这意味着如果要继续进行还原反应,气体中的全部 CO 就不可能转化为 CO2。例如,在 800 摄氏度时,与 FeO 和固体铁接触的平衡气体混合物含有约 65% 的 CO 和 35% 的 CO2。如果气体中的 CO2 含量在此温度下超过此值,则铁往往会被氧化回 FeO。因此,为了发生这些反应,如图 1 所示,在每个步骤中气体中的 CO 浓度必须达到最低限度,并且不可能通过这些反应将 CO 完全转化为 CO2。幸运的是,在这些温度下,还原反应产生的 CO2 在存在焦炭的情况下是不稳定的,并且 CO 基于反应 CO2 + C =2CO 再生,因此还原反应可以继续进行。值得注意的是,该反应与反应(iii)的结合对应于碳对FeO的“直接”还原(FeO + C =Fe + CO),这是一个强吸热反应。
氧化铁的还原也可以通过通过风口喷射的辅助燃料的部分燃烧产生的氢气来进行,以产生两种还原气体,CO 和氢气。当向鼓风中添加蒸汽以帮助控制熔炉时,也会产生氢气。热风中的氧气氧化碳生成CO是放热的,而焦炭还原水分生成CO和氢气(H2O + C =CO + H2)是强吸热的。
用氢还原氧化铁再次以顺序方式进行。反应为 (i) 3Fe2O3 + H2 =2Fe3O4 + H2O,(ii) FeO + H2 =Fe + H2O,和 (iii) Fe3O4 + H2 =3FeO + H2O。温度对这些反应平衡的影响如图 1 所示。虽然反应 (i) 略微放热,但反应 (ii) 和 (iii) 是吸热的。氢的存在,由于其小尺寸具有高扩散性,显着降低了高炉气体的密度和粘度,特别是在高温下,增强了低还原性原材料的还原。气相中不同组分之间可以发生水煤气变换反应(CO2+H2=H2O=CO),使含氢气体和含碳气体达到平衡。
从图 1 可以明显看出,通过炉子的气体不能与焦炭中的碳平衡,同时也不能与下降炉料中的氧化铁平衡。在大约 800 摄氏度以上,气体与碳的反应比与氧化物的反应更快,焦炭和气相之间的平衡可能相当接近。对操作炉中气体的温度和成分的测量表明,它们倾向于落在 800 摄氏度以上的 CO/CO2-C 线和 FeO/Fe 线之间,在 600 摄氏度和 800 摄氏度之间切断 FeO/Fe 线然后保持在或略高于 Fe3O4/Fe 线。在低于 600 ℃ 的温度下,非常快速的气流使与固体反应的时间很短,并且气体中的 CO 含量远远超过与焦炭平衡的含量。
如果氧化铁与其他氧化物发生化学结合,则其在 BF 中的活性会降低。这意味着氧化铁更难还原,所需的 CO/CO2 比值高于此处通常考虑的值。例如,对于硅酸亚铁,在 700 摄氏度下还原所需的最小 CO/CO2 比率将从大约 1.5 增加到大约 22,即以含碳气体为基础,从大约 60% CO 增加到几乎 96% CO。由于复合氧化物更难还原,因此还原需要更高的温度,因此在渣形成之前用 CO 实现的还原量会减少。这意味着由于炉下部所需的还原量增加,焦炭率增加。
炉膛中的反应
其他氧化物的还原 – 如果产物是纯金属,则比氧化铁更稳定的氧化物(如氧化锰和二氧化硅)不会在 BF 中发生还原,因为反应 MnO + CO =Mn + CO2 在平衡时具有非常接近的 CO 百分比到 100%。即,还原效率极低,还原极少量的锰需要大量气体。二氧化硅的情况更为极端,因为它是一种非常稳定的氧化物。然而,通过将锰和硅溶解在铁中,反应 MnO + CO =Mn(溶解在铁中)+ CO2 和 SiO2 + 2CO =Si(溶解在铁中)+ 2CO2 稍微向右移动,因此有一个锰和硅在金属和熔渣之间的分布是熔渣成分和温度的函数。由于这两种元素的还原是吸热的,因此热金属中每种元素的含量都随着温度的升高而增加,并且反应的程度在某种程度上是通过控制炉膛温度来控制的。更重要的是,这些反应产生的CO2会发生Boudouard反应,导致焦炭消耗量增加。
锰的减少量显然还取决于带电矿石中的量。锰含量高达 2% 的矿石在铁水中的锰含量远高于正常的锰含量,因此每生产一吨铁水的焦炭率更高。由炉料不稳定或温度变化引起的硅“波动”也可能产生另一个严重影响,因为硅被还原成热金属,它将从炉渣中耗尽,因此增加碱度比并改变熔点和炉渣的流动性有时非常显着。
一氧化硅 (SiO) 形成的影响 – For several years it was considered that silica and manganese oxide are reduced directly from the slag by reaction with carbon in iron according to the reactions (i) SiO2 (slag) + 2C =Si + 2CO (g), and MnO (slag) + C =Mn + CO (g). It was thought that liquid iron droplets picked up silicon as they passed through the slag phase and on into the hearth. Various studies however, have shed new light on these reactions and also those involving sulphur. Several laboratory studies together with plant data have shown that at the temperature of the combustion zone, around 2,000 deg C, SiO gas is produced during the combustion of coke by the reaction SiO2 (coke ash) + CO =SiO (gas) + CO2. Combining this equation with the reaction for coke oxidation [CO2 + C (coke) =2CO] yields the overall reaction SiO2 (coke ash) + C (coke) =SiO (gas) + CO. While the presence of FeO in slag is likely to make SiO formation from slag very difficult, an additional source of silica is to be reduced silica-rich slag adhering to the coke particles. Following these reactions, silicon is transferred to iron droplets by reaction with SiO in the gas phase [SiO (gas) + C =Si + CO]. As iron droplets containing silicon pass through the slag layer, some of the silicon is oxidized by iron oxide and manganese oxide, and taken up by the slag [2FeO (slag) + Si =SiO2 (slag) + 2Fe, 2 MnO (slag) + Si =SiO2 (slag) + 2Mn.
Reduction of phosphorus – It is expressed by the reaction P2O5 + 5C =2P + 5CO; delta H =+995,792 kJ/kmol. The final reduction of phosphorus also takes place only at very high temperatures. However, unlike manganese and silicon the phosphorus is essentially completely reduced. For this reason, virtually all of the phosphorus in the charge is dissolved in the hot metal. The only means of controlling the phosphorus content of the hot metal is by limiting the quantity charged to the furnace.
Behaviour of sulphur – Sulphur is a troublesome element in BF operations since hot metal for steelmaking is to be low in sulphur. Levels of 0.035 % to 0.02 % are normal. The reaction by which sulphur is removed from liquid iron (S ) into the slag (S) is frequently represented by the reaction S + (CaO) + C =(CaS) + CO (g) Where sulphur (S ) and carbon (C ) in the metal react with lime (CaO) dissolved in the slag to form calcium sulphide in the slag and CO gas. The distribution of sulphur between slag and metal, (S) /S , is strongly influenced by a number of factors as described here. Increasing the basicity of the slag (CaO / SiO2 ratio) tends to raise the thermodynamic activity of CaO in the slag which pushes reaction to the right. An increased oxygen potential in the system pushes the reaction to the left. This is shown by rewriting the reaction S + (CaO) =(CaS) + O . This effect is very strong, and the presence of even small concentrations of FeO in the slag seriously limits the sulphur ratio (S) / S . Fortunately both silicon and carbon raise the thermodynamic activity of sulphur in hot metal by 5 times to 7 times. Accordingly, sulphur in hot metal is 5 times to 7 times easier to remove than it is from liquid steel which contains relatively little carbon and silicon.
Assuming sulphur in coke ash is present as CaS, the reaction which can occur with SiO in the combustion zone to form volatile SiS is CaS (coke ash) + SiO (gas) =CaO + SiS (gas). To a lesser extent, some CS gas can form by the reaction CaS (coke ash) + CO =CaO + CS (gas).
Sulphur transfer from these volatile species to liquid iron droplets then takes place within the bosh zone. A study has shown that when iron droplets containing silicon and sulphur are allowed to fall through the liquid slag, in the absence of MnO, the silicon content of the hot metal actually increases, and there is no transfer of sulphur. In the presence of MnO, silicon is removed from the metal by reaction and manganese transfers from slag to metal together with sulphur transfer from metal to slag take place. Based on the various results available, the sequence of reactions in the bosh and hearth are (i) the formation of SiO and SiS in the combustion zone, (ii) the transfer of silicon and sulphur to metal and slag droplets in the bosh, (iii) the oxidation of silicon by FeO and MnO in the slag as the iron droplets pass though the slag layer, and (iv) the desulphurization of metal droplets as they pass through the slag layer.
The sulphur distribution ratios found in the BF normally varies between 20 and 120. On the other hand experiments have shown that when metal and slag samples from BF are remelted in graphite crucibles at 0.1 MPa CO, the distribution ratio increases to between 120 and 220, depending on the slag basicity. This suggests that the oxygen potential of the system is higher than is to be expected for C-CO equilibrium in the furnace hearth. Hence, while thermodynamic conditions favour sulphur removal from the hot metal within the BF, kinetic considerations imply that the reaction can be more readily accomplished outside the furnace by external desulphurization.
Reaction of less abundant elements
In addition to the elements (that is Fe, P, Mn, Si, Al, Ca, Mg and S) which are normally considered in reporting the chemical composition of an iron-bearing material, there are a number of less abundant elements which undergo chemical reactions in the BF. Some of these can cause considerable operating difficulty and some can contaminate the product and make it unsuitable for certain steelmaking applications. The source of these elements is not only from natural iron ores, but also from waste materials such as scrap, steelmaking dust, and grindings etc., which are recycled through the BF. Some of the more important of these elements are arsenic, barium, chlorine, chromium, cobalt, copper, fluorine, lead, molybdenum, nickel, potassium, sodium, tin, titanium, vanadium and zinc.
Alkalis and zinc – Sodium, potassium and zinc, frequently called the ‘rogue elements’, can cause serious operating problems in the BF and are to be monitored and carefully controlled if stable conditions are to be maintained. The alkali metals enter the BF as the constituents of the gangue in the ore and also as a part of the coke ash, normally as silicates. In the stack of the furnace, the silicates react as per the equations (i) K2SiO3 + CO =2K + SiO2 + CO2 and (ii) Na2SiO3 + CO =2Na + SiO2 + CO2.
In the BF, the potassium reaction can take place above 500 deg C, while the sodium reaction occurs at around 600 deg C. At temperatures of around 900 deg C, the alkali metals are above their boiling point so they join the gas phase. However, as these gases start to rise up the furnace, the metal becomes unstable with respect to other compounds which can form and cyanides, oxides, and carbonates all start to precipitate from the gas phase as very fine fumes or mists, since the cyanides are liquid over a wide temperature range. These fine particles of solid and liquid can deposit on the iron ore particles, the coke, and the furnace wall, with some, of course, being swept out with the BF gas and being captured in the dust catching system. Particularly the liquid alkali compounds can penetrate the brick lining of the furnace and cause serious deterioration and spalling. As well, these compounds can build upon the wall and cause scaffolding, hanging, and slipping.
The alkalis which land on the iron and coke are carried to the lower part of the furnace. There, they are again reduced to the metal which rises up the stack as a gas, forms the same alkali compounds, and repeats the cycle, joining new material in the process. The reduction needs carbon, increasing the coke rate, and cooling the furnace, and the recycling material can build up to the point where it degrades the coke in the furnace, causing it to break into small pieces and increasing the reactivity of the coke to CO2.
This increased reactivity can again reduce the temperature of the furnace and decrease the heat efficiency of the whole system. The high concentration of alkalis in the furnace also affects the strength and reduction characteristics of the iron bearing materials, causing dramatic swelling and catalyzing carbon deposition on the pellets. These deleterious reactions with both the coke and the ore can have serious impacts on the gas permeability in the furnace and on the stability of the BF operation.
Fortunately, the alkali oxides are very basic oxides and can be fluxed with SiO2 in acid slags and removed from the furnace. Normally, decreasing the slag basicity can carry increasing quantities of alkali away in the slag. This is in direct contrast to sulphur removal, where increasing the slag basicity increases the sulphur removal. When majority of desulphurizing takes place in the BF, there is a conflict between the attainment of low sulphur and removal of alkalis and the basicity of the furnace is carefully controlled to balance both the problems. With external desulphurization, this is no longer a problem and the furnace can normally be burdened to minimize alkali attack.
Zinc normally originates in steelmaking off-gas dust from furnaces using galvanized scrap which in some fashion has been recycled to the BF. Occasionally, the zinc content of iron ores or coal ash can be also a considerable source. Behaving not unlike sodium, zinc is reduced from the oxide or ferrite at around 600 deg C, forms a vapour which subsequently forms oxides or carbonates that can react with the sidewalls or be carried down the furnace on coke or ore to be reduced and further cycled, consuming coke at each turn. Zinc which escapes as a fume in the gas stream, enters the BF filter-cake, making it unsuitable to recycle if present in a high enough percentage. Unlike the alkalis, zinc is not captured to any extent in the slag and can only effectively be removed by decreasing the input and allowing the recycling vapour to slowly leave through the gas phase.
Clearly, the best protection against alkali metals and zinc is to ensure that the absolute minimum is part of the BF feed. Because of the tendencies of these elements to circulate in the furnace, they are unseen and unknown consumers of coke and cause refractory, ore and coke problems. Unfortunately, the symptoms of the problem are not always evident until the problem is of fairly major proportions and then needs fairly drastic measures, such as eliminating certain feed materials, to affect a solution.
Lead and titanium – Lead is seldom a problem in the BF but occasionally enough can enter a BF through the ore or sinter to cause a problem. Lead is very easily reduced in the iron BF and falls to the bottom of the hearth which normally has a chilled hot metal layer which protects the hearth refractories. Lead has virtually no solubility in the hot metal so it forms a low melting point liquid pool on which the hot metal floats, and hence promotes more rapid attack on the hearth. In certain furnaces where this problem is known to occur, a second tap-hole, deeper than the iron notch, can be used to periodically tap the lead.
Titanium is an even more stable oxide than silica but in the BF it can form extremely stable carbides and nitrides. The titanium compounds, if present in small quantities can be effective in forming a light protective layer on the hearth surfaces and prolong the life of the hearth. For this reason, titani-ferrous ores are added judiciously to sinter mixes. However, at high concentrations, these same compounds can stiffen the slag while building up a heavy hearth layer, reducing the hearth capacity of the furnace. As with zinc, the best solution is to reduce the input and slowly eliminate the titanium from the furnace.
Arsenic – Arsenic is found in a number of iron ores. The behaviour of arsenic is very much like that of phosphorus, in that it is almost completely reduced and dissolves in the hot metal. It increases the fluidity of the hot metal and hence, it appears to increase the wear of refractories. It is not completely removed during the steel refining process and imparts brittleness to the finished steel.
Barium – Barium is chemically similar to calcium and occurs as a very basic oxide in some iron and manganese ores. It is not reduced in the BF but becomes part of the slag, increasing the slag basicity. It can cause difficulty in controlling the metal composition if the operator is not aware of its presence.
Chlorine – Chlorine occurs as alkali chlorides in several iron ores and as a contaminating compound in ores processed with sea water. Chlorine is also present in some coals used for injection. In the high temperature zone of the BF, these compounds are volatized and as they rise toward the top of the furnace they condense around cooling plates and cause corrosion. They can also condense in uptakes and down-comers where they form accretions which can eventually restrict the passage of the top gas, or react to form HCl (hydrochloric) acid and attack the gas cleaning system.
Chromium – Chromium is found in some ores and is reduced to a certain extent depending on the basicity of the slag and the operating temperature. Normally, around 50 % to 60 % of the chromium is reduced into the hot metal.
Cobalt, copper, and nickel – Cobalt, copper, and nickel occurs in several different ores. They are also present in iron-bearing tailings from the copper industry which are sometimes sintered and used in the BF to recover the iron. All three of these elements are reduced almost completely into the hot metal and are not oxidized in the steel refining process. As a result, in operations which produce steel which is to meet stringent ductility specifications, such ores cannot be used.
Fluorine – Fluorine compounds are found in several ores and behave somewhat like chlorine compounds. The ability of HF (hydrofluoric) acid to attack the gas cleaning system is well known.
Molybdenum and tungsten – Molybdenum and tungsten occur very rarely and only in such minute quantities that they can be ignored. If any compounds of these elements are present in the BF, they are at least 90 % reduced into the hot metal.
Tin – Tin is an element which enters the BF mostly by way of recycled materials such as scrap or sintered dusts. It is almost entirely reduced and dissolves in the hot metal.
Vanadium – Vanadium occurs and behaves in a manner somewhat similar to chromium. Around 50 % of the vanadium in the burden is reduced and enters the hot metal.
Selenium and tellurium – Selenium and tellurium, though somewhat rare, can be present in some raw materials. In their reactions they are similar to sulphur but possess an even greater tendency to remain with the metal.
Fluxes
Limestone charged to the furnace is calcined by the reaction CaCO3(s) =CaO(s) + CO2 (g); delta H =177.8 kJ at around 800 deg C. Magnesium carbonate in the dolomite of the charge is calcined by a similar reaction MgCO3(s) =MgO(s) + CO2(g); delta H =167.4 kJ at 50 deg C to 100 deg C lower temperatures. These reactions result in several undesirable conditions in the furnace. The first is that they need considerable heat and the second is that CO2 is released in the furnace. The additional CO2 raises the oxygen potential of the gases which inhibits the final step in the reduction of the iron ore, i.e., FeO to Fe. It also favours ‘solution’ of carbon from the coke by the equation CO2 (g) + C(s) =2CO (g).
A considerable improvement in the furnace operations is achieved when ‘self-fluxing’ agglomerates of iron-ore concentrates are the principal iron-bearing charge to the furnace. Limestone and dolomite can be added to the feed of sintering machines and pelletizing furnaces. When the sinter is fired and the pellets are indurated, the fluxes are calcined and reacted with iron oxides to form calcium-ferrites and other more complex compounds. The CaO and MgO carried into the BF by these agglomerates are then free of CO2.
Slags
高炉渣的基本原理很复杂。氧含量约为 40%,是炉渣中最大的单一元素。 Slag is, hence, an oxide system and ionic in nature.形成高炉渣基础的氧化物系统是石灰-二氧化硅-氧化铝 (CaO-SiO2-Al2O3) 系统,由于渣中存在一定百分比的 MgO 而进行了改性。 Fig 4 show phase diagram of CaO-Al2O3-SiO2-10 % MgO system.由于 BF 工艺的性质,熔渣形成是一个多步骤过程,涉及成分和温度的显着变化。 The four primary components of slags form numerous compounds which result in a wide range of chemical and physical properties. The lesser components of slag are of particular interest with respect for hot metal chemistry and furnace control, and add to the complexity of the physico-chemical properties of the slag.
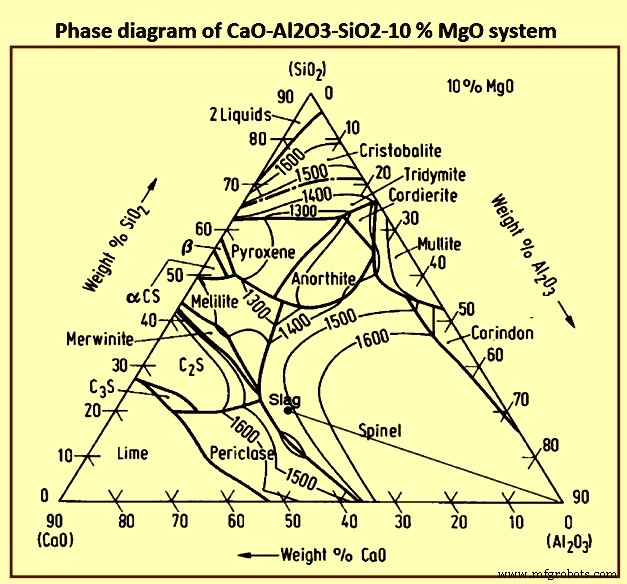
Fig 4 Phase diagram of CaO-Al2O3-SiO2-10 % MgO system
Slags with compositions in the region of 40 % SiO2, 48 % CaO, and 12 % A12O3 have low melting points, i.e., 1,300 deg C, and are appropriate for the control of sulphur and silicon in the hot metal. Frequently 6 % to 10 % MgO is used in place of an equivalent quantity of CaO to lower the viscosity of the slag. Small quantities of MnO, FeO, Na2O, and K2O etc. help to lower the melting point of the slag.
Essentially there are two slags in the furnace. The first is the ‘primary, or bosh, or early’ slag which is formed principally from the gangue constituents in the ores and agglomerates and CaO and MgO from the calcined fluxes, or the self-fluxing portions of the agglomerates. This slag is relatively basic compared to the final slag and contains some iron oxide. The ‘final or hearth’ slag is formed by the union of the early slag with constituents of the coke ash which are freed from the coke when it is burned before the tuyeres. This final slag continues to have its composition modified as it passes down into the hearth and mixes with liquid iron which also is flowing down into the crucible. There is an adjustment in the silica content of the slag, iron oxide can be reduced from it and it can absorb sulphur from the coke and liquid iron.
The formation of slags in the slag-formation zone is very furnace specific due to the impact of burden properties and furnace operation.熔渣形成区开始于粘性区,炉料开始软化,并继续下降到风口高度以下。因此,渣形成区包括内聚区、活性焦炭区、死区和滚道。在成渣区上部形成的熔渣称为“炉渣”或“初级”熔渣,离开底部区域的熔渣称为“炉膛”熔渣。 The Primary slag is normally assumed to be made up of all burden slag components including the iron oxides not reduced in the granular zone, but does not include the ash from the coke or injected coal.由于从气体中吸收焦炭灰和煤灰、硫和硅,以及氧化铁的还原,渣成分在炉内下降时发生变化。随着炉渣下降到风口高度,炉渣的温度增加了大约 500 摄氏度。这些成分和温度的变化会显着影响渣的物理性质,特别是液相线温度和粘度。
The slag produced in slag formation zone collects in the slag layer in the hearth zone, filling the voids in the hearth coke and ‘floating’ on the hot metal layer.铁水通过熔渣层到达铁水层。 The high surface area between the hot metal and slag as the hot metal passes through the slag layer enhances the kinetics of the chemical reactions.这些反应导致热金属化学发生相当大的变化。 In particular the (Si) and (S) contents prior to entering the slag layer are much higher than those in the hot metal layer.
制造工艺


