纳米粒子毒性对其物理和化学性质的依赖性
摘要
对纳米颗粒 (NP) 合成方法的研究、对其特性的分析以及对其应用新领域的探索处于现代纳米技术的前沿。工程水溶性纳米颗粒的可能性为其在各种基础和应用生物医学研究中的应用铺平了道路。目前,NPs 被用于诊断遗传和自身免疫性疾病、恶性肿瘤和许多其他疾病的众多分子标志物的成像。 NPs 还用于将药物靶向递送至组织和器官,具有可控的药物释放和积累参数。此外,还有一些使用 NP 作为活性成分的例子,例如光动力疗法中的光敏剂,以及通过 NP 掺入和加热来破坏热疗肿瘤。然而,NPs 对生物体的高毒性是阻碍其在体内使用的强大限制因素。目前在细胞培养模型中进行了关于 NPs 毒性作用的研究,旨在确定其有害作用的靶点和机制;在动物模型中研究 NP 转运、积累、降解和消除的模式。本综述系统化并总结了关于 NP 对生命系统的毒性机制与其理化性质相关的现有数据。
背景
国际标准化组织将纳米颗粒 (NP) 定义为一维、二维或三维尺寸在 1 到 100 纳米范围内的结构。除了大小,NPs 还可以根据它们的物理参数进行分类,例如电荷;化学特性,例如 NP 核或壳的组成;形状(管、薄膜、棒等);来源:天然纳米颗粒(火山灰、病毒颗粒等中含有的纳米颗粒)和人工纳米颗粒,是本次综述的重点。
纳米粒子已广泛应用于电子、农业、纺织生产、医学和许多其他行业和科学。然而,NP 对生物体的毒性是限制它们在疾病治疗和诊断中使用的主要因素。目前,研究人员经常面临纳米颗粒的积极治疗效果与其毒性相关副作用之间的平衡问题。在这方面,选择合适的实验模型来估计体外(细胞系)和体内(实验动物)之间的毒性至关重要。在体外模型中更容易分析 NP 对单个细胞成分和单个组织的毒性作用,而体内实验则可以估计单个器官或整个身体的 NP 毒性。此外,NPs 可能的毒性作用取决于它们的浓度、它们与生物体相互作用的持续时间、它们在生物体液中的稳定性以及在组织和器官中的积累能力。开发可用于人类疾病诊断和治疗的安全、生物相容性纳米颗粒,只能基于对纳米颗粒毒性的所有因素和机制之间的相互作用的全面了解。
纳米粒子的医学应用
在医学中,NPs 可用于诊断或治疗目的。在诊断中,它们可以用作检测生物分子和病原体的荧光标记,以及在磁共振和其他研究中用作造影剂。此外,NPs可用于靶向递送药物,包括蛋白质和多核苷酸物质;在光动力疗法和热破坏肿瘤以及假体修复中 [1,2,3,4,5,6]。部分类型的纳米颗粒已成功应用于临床药物递送和肿瘤细胞成像[7,8,9]。
最近使用金纳米粒子的例子越来越多。它们已被证明是化疗药物和其他药物的有效载体。金纳米粒子具有高度的生物相容性;然而,虽然金作为一种物质对生物物体是惰性的,但不能说金纳米粒子也是如此,因为目前还没有关于不存在延迟毒性作用的结论性数据 [10]。除了金纳米粒子,那些基于胶束、脂质体 [11] 和带有“捕获分子”[12] 聚合物的纳米粒子已经被用作药物载体。单壁和多壁纳米管是用于药物输送的 NPs 的很好例子。它们适用于附着各种官能团和分子进行靶向递送,其独特的形状使它们能够选择性地穿透生物屏障[13]。使用 NPs 作为药物的载体增强了递送的特异性并减少了获得和维持治疗效果所需的 NPs 的最小量,从而降低了最终的毒性。这对于剧毒和短效化疗和放疗药物尤为重要[14]。
量子点 (QD) 构成了另一组具有很高临床应用潜力的 NP。 QD 是大小从 2 到 10 纳米的半导体纳米晶体。它们在不同光谱区域(包括红外光谱区域)发出荧光的能力 [15],使其适用于标记和成像细胞、细胞结构或病原体生物制剂,以及细胞、组织和整个身体的各种过程。 16,17,18],具有重要的诊断意义 [19, 20]。基于超顺磁性氧化铁的纳米颗粒被有效地用作磁共振断层扫描 (MRT) 中的造影剂,用于对肝脏、骨髓和淋巴结组织进行成像 [21]。还有一个例子是用磷脂功能化的放射性标记单壁碳纳米管标记含有整合素的肿瘤,并随后在小鼠实验中通过正电子发射断层扫描进行检测[22]。
纳米颗粒也被用于设计生物传感器,包括基于碳纳米管的那些用于测量葡萄糖水平 [23]、检测特定 DNA 片段和区域 [24] 以及识别细菌细胞 [25]。
银(或含银)NPs 具有抗菌和细胞抑制作用;因此,它们被广泛用于医学,例如用于治疗绷带、手术器械、假肢和避孕药 [13, 22]。据报道,银纳米颗粒可作为化妆品行业有效且安全的防腐剂[26]。
然而,即使已证明在医学中使用许多化学成分是安全的,NPs 仍可能具有高毒性。毒性作用可能是由它们独特的物理和化学特性引起的,这些特性是与生命系统相互作用的特定机制的基础。总的来说,这决定了研究纳米颗粒潜在毒性作用的原因和机制的重要性。
纳米粒子毒性机制
纳米颗粒的毒性在很大程度上取决于其物理和化学特性,例如它们的大小、形状、比表面积、表面电荷、催化活性以及是否存在外壳和表面活性基团。
NPs 的小尺寸使它们能够穿透上皮和内皮屏障进入淋巴和血液,由血液和淋巴流携带到不同的器官和组织,包括大脑、心脏、肝脏、肾脏、脾脏、骨髓和神经系统 [27, 28],或者通过胞吞作用机制转运到细胞中,或者简单地通过细胞膜扩散到细胞中。纳米材料还可以通过摄入增加进入血流的途径 [29, 30]。一些纳米材料可以穿透皮肤 [31, 32],甚至更大的微粒在弯曲时可以穿透皮肤 [33]。纳米颗粒由于尺寸小,可以通过炎症部位的内皮、上皮(例如肠道和肝脏)、肿瘤或穿透微毛细血管 [34]。模拟 NPs 对身体毒性作用的实验表明,NPs 通过增强血小板聚集引起血栓形成 [35]、上呼吸道和下呼吸道炎症、神经退行性疾病、中风、心肌梗塞和其他疾病 [36,37,38] ]。请注意,NPs 不仅可以进入器官、组织和细胞,还可以进入细胞器,例如线粒体和细胞核;这可能会彻底改变细胞代谢并导致 DNA 损伤、突变和细胞死亡 [39]。
量子点的毒性已被证明与其核心中所含金属的游离离子(如镉、铅和砷)在被环境介质氧化时的泄漏直接相关。量子点可能被线粒体吸收并导致细胞器的形态变化和功能障碍 [40]。镉基量子点进入细胞并形成游离 Cd 2+ 离子引起氧化应激[41, 42]。
最近的研究表明,肺组织与约 50 nm 大小的 NP 接触会导致 I 型肺泡细胞膜穿孔,从而导致 NP 进入细胞。这反过来又会导致细胞坏死,正如乳酸脱氢酶的释放所证明的那样 [43]。有证据表明 QD 渗透会增加细胞膜的流动性 [44]。另一方面,膜脂过氧化引起的活性氧(ROS)的形成可能导致膜的柔韧性丧失,以及异常高的流动性,不可避免地导致细胞死亡。
NPs 与细胞骨架的相互作用也可能对其造成损害。例如,TiO2 NPs 会诱导微管蛋白的构象变化并抑制其聚合 [45],从而干扰细胞内运输、细胞分裂和细胞迁移。在人脐静脉内皮细胞(HUVECs)中,细胞骨架的损伤阻碍了连接细胞骨架和细胞外基质的配位粘附复合物的成熟,从而干扰了血管网络的形成[46]。
此外,NP的细胞毒性可能会干扰细胞分化和蛋白质合成,以及激活促炎基因和炎症介质的合成。需要特别注意的是,正常的保护机制不会影响NPs;巨噬细胞对大聚乙二醇化纳米粒子的吸收比对小纳米粒子的吸收更有效,这会导致 NP 在体内积累 [47]。超顺磁性氧化铁纳米颗粒已被证明可以干扰或完全抑制干细胞的成骨分化,并激活信号分子、肿瘤抗原等的合成 [48, 49]。此外,NPs 与细胞的相互作用增强了负责形成溶酶体的基因的表达 [50],扰乱了它们的功能 [51],并抑制了蛋白质合成 [52, 53]。一项关于不同成分的 NPs 对肺上皮细胞和人类肿瘤细胞系的毒性作用的研究表明,NPs 会刺激炎症介质的合成,例如白细胞介素 8 [54]。 Park 研究了体外和体内促炎细胞因子的表达,发现白介素 1 β (IL-1β) 和肿瘤坏死因子 α (TNFα) 的表达因硅纳米颗粒而增强 [55]。
氧化以及各种酶对 NPs 的外壳和表面的作用,导致它们降解和释放自由基。除了表现为酶的氧化和失活、诱变和化学反应干扰导致细胞死亡的自由基的毒性作用外,NPs 的降解还会导致其自身功能的改变或丧失(例如,磁矩的丧失)以及荧光光谱和传输或其他功能的变化)[56, 57]。
总之,NP细胞毒性最常见的机制如下:
- 1.
NPs可能通过形成ROS和其他自由基引起氧化;
- 2.
NPs可能会通过穿孔破坏细胞膜;
- 3.
NPs损伤细胞骨架成分,干扰细胞内转运和细胞分裂;
- 4.
NPs干扰转录并损伤DNA,从而加速诱变;
- 5.
NPs损伤线粒体并干扰其代谢,导致细胞能量失衡;
- 6.
NPs干扰溶酶体的形成,从而阻碍自噬和大分子降解,引发细胞凋亡;
- 7.
NPs引起膜蛋白结构变化,干扰物质进出细胞的转运,包括细胞间转运;
- 8.
NPs通过干扰细胞代谢以及组织器官代谢的正常机制来激活炎症介质的合成(图1)。
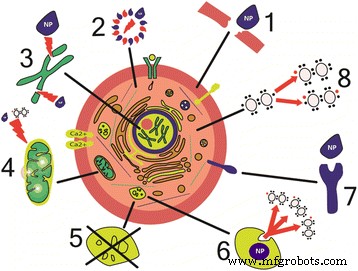
纳米粒子对细胞损伤的机制。 (1) 膜的物理损伤 [43, 67, 75]。 (2) 细胞骨架成分的结构变化 [45, 46]。 (3) DNA 转录紊乱和氧化损伤 [61, 62]。 (4) 线粒体损伤 [39, 40]。 (5) 溶酶体功能紊乱 [51]。 (6) 活性氧的产生[61]。 (7) 膜蛋白功能的干扰 [172]。 (8) 炎症因子及介质的合成[54, 55]
尽管纳米颗粒的毒性机制众多,但有必要根据纳米颗粒的理化性质对纳米颗粒的每种特定毒性作用的类型和机制进行确定和分类。
纳米粒子毒性与其理化性质的关系
NPs 的毒性被认为取决于它们的物理和化学特性,包括大小、形状、表面电荷、核壳的化学成分以及稳定性。特别是,Oh 等人最近对 307 篇描述 1741 个细胞活力相关数据样本的论文进行了数据元分析,分析了 CdSe 量子点毒性。已经表明,QD 纳米毒性与其表面特性(包括壳、配体和表面修饰)、直径、所使用的毒性测定类型和暴露时间密切相关 [58]。这些因素中哪个最重要由具体的实验任务和模型决定;因此,我们现在将分别考虑每个因素。
纳米粒子大小和毒性
NP 的大小和表面积起着重要作用,在很大程度上决定了 NP 与生命系统相互作用的独特机制。 NPs 的特点是非常大的比表面积,这决定了它们的高反应能力和催化活性。 NPs 的大小(从 1 到 100 纳米)与蛋白质球的大小(2 到 10 纳米)、DNA 螺旋的直径(2 纳米)和细胞膜的厚度(10 纳米)相当,这使得它们很容易进入细胞和细胞器。例如,霍等人。已经证明不大于 6 nm 的金 NPs 有效地进入细胞核,而大 NPs(10 或 16 nm)仅穿透细胞膜并且仅存在于细胞质中。这意味着几纳米大小的 NP 比 10 纳米或更大的 NP 毒性更大,后者无法进入细胞核 [59]。潘等人。已经追踪了金 NPs 的毒性对它们在 0.8 到 15 nm 范围内的大小的依赖性。已发现 15 纳米的纳米颗粒对成纤维细胞、上皮细胞、巨噬细胞和黑色素瘤细胞的毒性比 1.4 纳米的纳米颗粒低 60 倍。还值得注意的是,1.4-nm NPs 会导致细胞坏死(在它们加入细胞培养基后 12 小时内),而 1.2-nm NPs 主要导致细胞凋亡 [60]。这些数据不仅表明 NPs 可以进入细胞核,而且 NPs 的几何尺寸(1.4 nm)与 DNA 大沟的几何尺寸的对应关系使它们能够有效地与带负电荷的糖 - 磷酸盐 DNA 骨架相互作用,阻断转录[61, 62]。
此外,NP 的大小在很大程度上决定了 NP 如何与细胞和身体的运输和防御系统相互作用。反过来,这种相互作用会影响它们在体内的分布和积累的动力学。 [63] 的评论论文提出了理论考虑和大量实验数据,证明小于 5 nm 的 NPs 通常非特异性地克服细胞屏障,例如通过易位,而较大的颗粒通过吞噬作用、巨胞饮作用以及特异性和非特异性转运机制进入细胞.大约 25 nm 的 NP 大小被认为是胞饮作用的最佳选择,尽管这也很大程度上取决于细胞大小和类型 [63, 64]。体内实验表明,静脉给药后,小于 10 nm 的 NPs 会迅速分布在所有器官和组织中,而大多数较大的 NPs(50-250 nm)存在于肝脏、脾脏和血液中 [65]。这表明大 NP 被身体的特定防御系统识别并被单核吞噬细胞系统吸收,从而防止它们进入其他组织。此外,塔拉米尼等人。声称纳米颗粒的大小和形状影响过滤器官中金纳米颗粒的积累和排泄动力学,只有星状金纳米颗粒能够在肺中积累。他们还表明,NP 几何形状的变化并没有改善 NP 通过血脑屏障的能力 [66]。
大的比表面积确保了纳米颗粒在细胞表面的有效吸附。一项关于 100 至 600 nm 中孔硅颗粒对人类红细胞的溶血活性的研究表明了这一点 [67]。 100 nm 大小的颗粒被有效吸附在红细胞表面,而不会造成细胞破坏或细胞的任何形态变化,而 600 nm 颗粒使膜变形并进入细胞,导致红细胞破坏(溶血)[67]。
纳米粒子形状和毒性
NPs的特征形状是球体、椭球体、圆柱体、片状、立方体和棒状。 NP 毒性在很大程度上取决于它们的形状。这已在许多不同形状和化学成分的 NP 中得到证明 [68,69,70,71]。例如,球形纳米颗粒比纳米管和纳米纤维更容易发生内吞作用 [72]。与球形富勒烯相比,单壁碳纳米管已被发现能更有效地阻断钙通道[73]。
不同形状(针状、板状、棒状和球状)的羟基磷灰石纳米颗粒对培养的 BEAS-2B 细胞影响的比较表明,板状和针状纳米颗粒导致更大比例的细胞死亡。细胞比球形和棒状 NPs [74]。这部分是由于板状和针状 NPs 在直接接触时破坏细胞和组织的能力。胡等人。 [75] 在研究氧化石墨烯纳米片对哺乳动物细胞的损伤时获得了有趣的数据。这些 NPs 的毒性取决于它们的形状,允许它们物理损坏细胞膜。然而,发现它们的毒性随着培养基中胎牛血清浓度的增加而降低。这是由于氧化石墨烯纳米颗粒具有高吸附蛋白质分子的能力,蛋白质分子覆盖纳米颗粒表面,从而改变纳米颗粒的形状并在一定程度上防止细胞膜受损[75]。
纳米粒子化学成分和毒性
虽然纳米颗粒的毒性很大程度上取决于它们的大小和形状,但不应忽视其他因素的影响,如纳米颗粒的化学成分和晶体结构。比较 20 nm 二氧化硅 (SiO2) 和氧化锌 (ZnO) NPs 对小鼠成纤维细胞的影响表明它们的毒性机制不同。 ZnO NPs引起氧化应激,而SiO2 NPs改变DNA结构[76]。
NPs 的毒性确实很大程度上取决于它们的化学成分。已经表明,NPs 会发生降解,其程度取决于环境条件,例如 pH 值或离子强度。 NPs 与细胞相互作用的毒性作用的最常见原因是金属离子从 NP 核心泄漏。毒性还取决于 NPs 核心的组成。一些金属离子,例如银和镉,实际上是有毒的,因此会对细胞造成损害。其他金属离子,如 Fe 和 Zn,在生物学上是有用的,但在高浓度下,它们可能会破坏细胞通路,因此会导致高毒性。然而,这种影响可以降低,例如,通过用厚聚合物壳、二氧化硅层或金壳包覆 NP 核而不是短配体,或通过使用无毒化合物进行 NP 合成。另一方面,可以通过添加其他金属来改变核心的组成。这可以增强化学稳定性,防止NP降解和金属离子泄漏到体内[77]。
NPs 的毒性还取决于它们的晶体结构。已经使用人支气管上皮细胞系和具有不同晶格类型的氧化钛 NP 研究了晶体结构和毒性之间的关系。已经证明,具有金红石状晶体结构(棱柱形 TiO2 晶体)的 NPs 会导致 DNA 氧化损伤、脂质过氧化和微核的形成,这表明有丝分裂期间染色体分离异常,而具有锐钛矿状晶体结构的 NPs相同尺寸的(八面体 TiO2 晶体)是无毒的 [78]。应该注意的是,NP 晶体结构可能因环境而异,例如与水、生物流体或其他分散介质相互作用时。有证据表明,与水接触后,ZnS NPs 的晶格会重新排列成更有序的结构[79]。
纳米粒子表面电荷和毒性
纳米颗粒的表面电荷在很大程度上决定了纳米颗粒与生物系统的相互作用[80, 81]。
NP 表面及其电荷可以通过接枝不同带电的聚合物进行改性。 PEG(聚乙二醇)或叶酸通常用于提高 NP 细胞内摄取和靶向特定细胞的能力 [82]。也有报道合成含有功能性 NH2 或 SH 基团的生物相容性 TiO2 纳米粒子 [83]。其他物质,如甲氨蝶呤、聚乙烯亚胺和葡聚糖,也被用于修饰 NP 表面及其电荷 [84]。
与带负电和中性 NPs 相比,带正电 NPs 的高毒性可以通过它们容易进入细胞的能力来解释。这是由带负电荷的细胞膜糖蛋白和带正电荷的 NPs 之间的静电吸引力引起的。带负电和带正电的聚苯乙烯 NPs 对 HeLa 和 NIH/3T3 细胞的细胞毒作用的比较表明,后者的 NPs 毒性更大。这不仅是因为带正电的 NPs 能更有效地穿透细胞膜,还因为它们与带负电的 DNA 结合得更牢固,造成其损伤,从而延长细胞周期的 G0/G1 期。带负电的 NP 对细胞周期没有影响 [85]。带正电和带负电的金纳米颗粒也得到了类似的结果,正纳米颗粒被细胞吸收的量更大,比负纳米颗粒更快,毒性更大[86]。
带正电荷的 NPs 具有增强的调理能力,即从血液和生物体液中吸附促进吞噬作用的蛋白质,包括抗体和补体成分 [87]。吸附的蛋白质,称为蛋白质冠,可能会影响纳米颗粒的表面性质。例如,它们可能会改变纳米颗粒的表面电荷、聚集特性和/或流体动力学直径。此外,蛋白质在纳米颗粒表面的吸附会导致其构象发生变化,这可能会降低或完全抑制被吸附蛋白质的功能活性。蛋白冠主要由主要的血清蛋白组成,如白蛋白、纤维蛋白原和免疫球蛋白 G,以及其他效应分子、信号分子和功能分子 [88, 89]。与 NP 结合会改变蛋白质结构,从而导致其酶活性丧失、生物过程紊乱以及有序聚合结构(例如淀粉样蛋白原纤维)的沉淀 [90]。这可能导致各种疾病,例如淀粉样变性。体外实验表明,涂有亲水聚合物的量子点加速了人β2微球蛋白原纤维的形成,然后在颗粒表面排列成多层结构;这导致NP表面的蛋白质浓度局部增加、沉淀和低聚物的形成[91]。
徐等人。开发了一种通过表面的各种修饰将 NP 电荷从负变为正的方法。例如,聚合物 NP 被 pH 敏感聚合物改性,因此,它们在中性介质中带负电,在酸介质中,在 pH 5-6 时获得正电荷 [92]。该技术可以显着提高细胞对 NP 的摄取率,这可用于向肿瘤细胞输送药物。对表面修饰氧化铈纳米颗粒对 H9C2、HEK293、A549 和 MCF-7 细胞的细胞毒性的估计表明,通过使用不同的聚合物使纳米颗粒带正电荷或负电荷或中性,可以获得基本不同的生物学和毒性作用。具体而言,带正电荷和中性 NPs 以相同的速率被所有细胞类型吸收,而带负电荷的 NPs 主要积聚在肿瘤细胞中 [93]。因此,修饰NP电荷可以控制它们的定位和毒性,可用于开发有效的向肿瘤递送化疗药物的系统。
纳米粒子壳和毒性
将壳应用到 NPs 表面是改变它们的光学、磁性和电学性质所必需的;它用于通过降低纳米颗粒的聚集能力、增加其稳定性等来提高纳米颗粒的生物相容性和在水和生物体液中的溶解度。 因此,外壳降低了纳米颗粒的毒性,并为它们提供了与不同类型细胞选择性相互作用的能力,生物分子。此外,壳显着影响NP的药代动力学,改变NP在体内的分布和积累模式[94]。
如上所述,NP 毒性在很大程度上与自由基的形成有关 [40, 57, 95, 96]。然而,外壳可以显着减轻或消除这种负面影响,并稳定 NPs,增加它们对环境因素的抵抗力,减少有毒物质的释放,或使它们具有组织特异性 [97]。例如,Cho 等人。通过用凝集素包被修饰的聚合物纳米颗粒。修饰后的NPs选择性地与表面呈递唾液酸分子的肿瘤细胞结合,这使得NPs适用于特异性标记癌细胞[98]。
NP 表面可以用有机和无机化合物改性,例如聚乙二醇、聚乙醇酸、聚乳酸、脂质、蛋白质、低分子量化合物和硅。多种修饰剂使得在纳米颗粒表面形成复杂系统以改变纳米颗粒的性质及其特定的运输和积累成为可能。
涂有合成聚合物外壳的纳米颗粒用于递送抗原,从而作为增强免疫反应的佐剂。这允许获得针对抗原的疫苗,这些抗原是强天然非特异性细胞免疫的靶点[99]。
壳通常用于提高 QD 的增溶和降低毒性,因为它们的金属核是疏水的,主要由有毒重金属组成,如镉、碲和汞。壳增加了 QD 核的稳定性并防止其脱盐和氧化或光解降解。这反过来又减少了量子点核心外金属离子的泄漏,从而降低了量子点的毒性[100,101,102]。
纳米粒子毒性研究
在过去的 20 年中,NPs 的使用得到了极大的扩展,并为纳米毒理学奠定了基础,这是一门研究 NPs 对生物和生态系统潜在毒性影响的新科学。纳米毒理学的总体目标是制定合成安全纳米颗粒的规则 [103]。这需要一种全面、系统的方法来分析纳米颗粒的毒性及其对细胞、组织、器官和整个身体的影响。
研究各种物质对生命系统影响的常规方法有两种,它们也适用于 NP 毒性作用:模型细胞系的体外实验和实验室动物的体内实验。我们在此不考虑估计 NP 毒性的第三种可能方法,即计算机模拟,因为对 NP 毒性作用的途径和机制还不够了解,因此计算机模型无法预测 NP 与生物之间相互作用的后果。范围广泛的NPs,具有足够的可靠性。
用于研究 NP 毒性的细胞培养和动物实验模型都有其特定的优点和缺点。前者可以更深入地了解毒性的分子机制和确定 NP 的主要靶点;然而,没有考虑NPs在体内的分布模式及其向不同组织和细胞的运输。在动物实验中对 NP 毒性的研究可以估计 NP 作用在体内的延迟效应。 However, the general pattern of toxicity manifestations becomes so complicated that it is impossible to determine which of them is the primary cause of the observed effect and which are its consequences.
Study of Toxicity in Cell Cultures
Many studies of NP toxicity are carried out in primary cell cultures serving as models of various types of human and animal tissues. In some cases, tumor cell lines are used, e.g., for estimating the toxic effects of NPs used in cancer chemotherapy. The type of cells is selected according to the potential route by which NPs enter the body. This may be oral uptake (mainly by ingestion), transdermal uptake (through the skin surface), inhalation uptake of NPs contained in the breathing air, or intentional NP injection in clinic. Intestinal epithelium cells (Caco-2, HT29, and SW480) are often used in experimental models for studying the toxicity of ingested NPs (Table 1). In these models, the kinetics of NP uptake by cells and the viability of cells upon the NP uptake are studied.
The NPs that serve as carriers of drugs or contrast agents, or those used for imaging, are administered by injection. The toxicity of these NPs is studied in primary blood cell cultures. Most commonly, hemolysis, platelet activation, and platelet aggregation are estimated. In addition to primary blood cell cultures, cultured HUVECs, mesenchymal stem cells, mononuclear blood cells, and various tumor cell lines (HeLa, MCF-7, PC3, C4-2, and SKBR-3) are used (Table 2).
The toxicity of inhaled NPs is studied using the cell lines modeling different tissues of the respiratory system, e.g., A549 and C10 cells of pulmonary origin, alveolar macrophages (RAW 264.7), various epithelial cells and fibroblasts (BEAS-2B, NHBE, 16-HBE, SAEC), as well as human monocytes (THP-1) (Table 3).
The toxicity of NPs that enter the body transdermally is usually studied in keratinocytes, fibroblasts, and, more rarely, sebocytes (cells of sebaceous glands) (Table 4).
Co-cultured Cell Lines and 3D Cell Cultures
Although the majority of in vitro nanotoxicity studies are carried out on cell monocultures, studies using two other approaches are increasingly often reported in the literature. One of them is co-culturing of several types of cells; the other is the use of 3D cultures. The rationale for these approaches is the need for more realistic models of mammalian tissues and organs. For example, co-cultured Caco-2 epithelial colorectal adenocarcinoma cells and Raji cells (a lymphoblast cell line) have served as a model of the human intestinal epithelium in experiments on the toxicity of silver NPs [104]. A co-culture of three cell lines derived from lung epithelial cells, human blood macrophages, and dendritic cells has been used as an experimental model in a study on the toxic effects of inhaled NPs [105]. A model of skin consisting of co-cultured fibroblasts and keratinocytes has been suggested [106].
It is known that the cell phenotype, as well as cell functions and metabolic processes, is largely determined by the complex system of cell interactions with other cells and the surrounding extracellular matrix [107]. Therefore, many important characteristics of cells with an adhesive type of growth in a monolayer culture substantially differ from those of the same cells in the living tissue; hence, conclusions from many experiments on the NP toxic effects on cells growing in a monolayer are somewhat incorrect [108]. Experimental 3D models of tissues and organs have been used for analysis of NP toxicity and penetration into cells in several published studies. For example, there are 3D models based on polymer hydrogels [109] and models constructed in special perfusion chambers containing a semipermeable membrane to which the cells are attached.李等人。 and Lee et al. [110, 111] used multicellular spheroids about 100 μm in size to obtain a 3D model of the liver and compare the toxicities of CdTe and Au NPs in experiments on this model and a monolayer culture of liver cells [111]. The results obtained using the 3D model were more closely correlated with the data obtained in experiments on animals, which indicates a considerable potential of this approach for adequate and informative testing of NP toxicity.
In vivo Study of Nanoparticle Toxicity
In addition to the study of multilayered and 3D cell cultures, the behavior of NPs in the living body is being extensively studied. Since these studies are focused on the biomedical applications of NPs, the NP toxicity for living organisms remains an important issue. Although NPs are highly promising for various clinical applications, they are potentially hazardous. This hazard cannot be estimated correctly in vitro, following from the comparison of the in vivo and in vitro effects of NPs.
Titanium dioxide (TiO2) particles are among the most widely used NPs, in particular, in environment protection measures. Therefore, it was exceptionally important to estimate their toxicity in the case of a 100% bioavailability, namely, in experiments with their intravenous injection to experimental animals. This study has been performed by Fabian et al. [112]. Experimental animals (rats) were injected with a suspension of TiO2 NPs at a dose of 5 mg/kg, and their biodistribution, as well as the general condition of the animals, was monitored. The results have shown that the animals exhibit no signs of ailment or disorder, nor is inflammation or another manifestation of a toxic effect observed, within 28 days. This suggests that TiO2 NPs are relatively harmless.
Silver NPs are another example of NPs potentially useful in medicine, owing to their antimicrobial activity. Their toxicity and biodistribution were analyzed in an experiment where CD-1 mice were intravenously injected with 10 mg/kg of silver NPs of different sizes (10, 40, and 100 nm) coated with different shells. Although each type of NPs was found to cause toxic damage of tissues, larger particles were less toxic, probably, due to their lower penetration capacity [113]. Asare et al. [114] estimated the genotoxicity of silver and titanium NPs administered at a dose of 5 mg/kg. They have found that silver NPs cause DNA strand breaks and oxidation of purine bases in the tissues examined. Gold nanoparticles have a similar effect [115]. They have been shown to be toxic for mice, causing weight loss, decrease in the hematocrit, and reduction of the red blood cell count.
Targeted drug delivery is one of the most important applications of NPs. In this case, it is also paramount to know their toxic properties, because the positive effect of their use should prevail over the negative one. Kwon et al. [116] have developed antioxidant NPs from the polymeric prodrug of vanillin. Their study has shown that the NPs have no toxic effect on the body, specifically the liver, at doses lower than 2.5 mg/kg. Similar results have been obtained for gelatin NPs modified with polyethylene glycol, which are planned to be used for targeted delivery of ibuprofen sodium salt [117]. The NPs have proved to be nontoxic at the dose that is necessary for effective drug delivery (1 mg/kg), which has been confirmed by measuring the inflammatory cytokine levels in the animals studied, as well as histological analysis of their organs.
Quantum dots are among the NPs that are most promising for medical applications (Fig. 2). However, they are potentially hazardous for human health, because they exhibit various toxic effects in both in vitro and in vivo experiments [118,119,120,121,122].
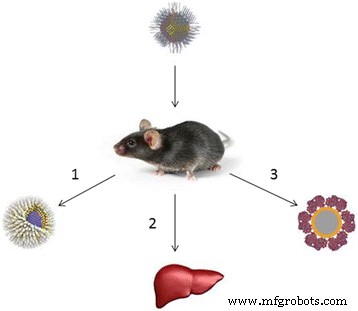
The possible reasons why quantum dots may be nontoxic in animal models. (1) The shell prevents the leakage of heavy metals into the body [129, 135]. (2) Quantum dots are localized in the liver and subsequently eliminated from the body [135, 173]. (3) The protein crown around quantum dots protects the body from heavy metals [132, 174]
Toxic effects of QDs in vivo are usually studied in experiments on mice and rats [123]. A study on the toxicity of cadmium-based QDs for mice showed that QDs were distributed throughout the body as soon as 15 min after injection to the caudal vein, after which they accumulated in the liver, kidneys, spleen, red bone marrow, and lymph nodes. Two years after the injection, fluorescence was mainly retained in lymph nodes; in other organs, no QDs were detected [124]. It should be also noted that the fluorescence spectrum was shifted to the blue spectral region because of the destruction of the QD shell and changes in the shape, size, and surface charge of the QDs. This, however, occurred rather slowly, because the QDs were found to be nontoxic after their injection at the doses at which pure cadmium ions would have had a lethal effect. Similar results were obtained by Yang et al. [125]. Zhang et al. [95] showed that CdTe QDs predominantly accumulated in the liver, decreasing the amount of antioxidants in it and inducing oxidative stress in liver cells.
Cadmium and tellurium ions tend to accumulate in various organs and tissues upon degradation and decay of the cores of CdTe/ZnS QDs. Experiments on mice have shown that cadmium predominantly accumulates in the liver, kidneys, and spleen, whereas tellurium accumulates almost exclusively in the kidneys [126]. Ballou et al. [127] found that cadmium-containing QDs coated with polymer shells of polyacrylic acid or different derivatives of polyethylene glycol had no lethal effect on experimental mice and remained fluorescent for 4 months. СdSe/ZnS NPs also had no detectable pathological effect on mice [128]; however, the absence of distinct signs of pathology still does not mean that the QDs are absolutely nontoxic.
Hu et al. [129] found that lead-containing QDs had no toxic effect on mice for 4 weeks; however, this was most probably because the QDs studied were coated with a polyethylene glycol shell.
Since heavy metals contained in QDs are a factor of their toxicity, several research groups suggested that heavy-metal-free NPs be synthesized. For example, Pons et al. [130] synthesized CuInS2/ZnS QDs fluorescing in the near-infrared spectral region (at a wavelength of about 800 nm) and supposed that this composition would make the QDs nontoxic for experimental animals. Comparison of the effects of CuInS2/ZnS and CdTeSe/CdZnS QDs on regional lymph nodes in mice showed that the lymph nodes were only slightly, if at all, enlarged upon injection of the QDs not containing heavy metals, whereas injection of the CdTeSe/CdZnS QDs induced a distinct immune response in them [130]. QDs in which silicon was substituted for heavy metals also had no toxic effect on mice [131].
Even QDs containing heavy metals are often found to be nontoxic. One of the possible explanations is that QDs are coated with the protein crown upon entering the living body; this crown shields their surface and protects cells against damage [132]. Usually, the proteins that are included in the NP molecular corona are major serum proteins, such as albumin, immunoglobulin G (IgG), fibrinogen, and apolipoproteins [133]. Molecular corona also can influence on the interaction of NPs with cells. Zyuzin et al. have demonstrated that, in human endothelial cells, the NP protein corona decreases the NP nonspecific binding to the cell membrane, increases the residence time of NP in early endosomes, and reduces the amount of internalized NPs [134].
However, even in the absence of direct signs of intoxication in experimental animals, it remains unclear whether the use of QDs in medicine is safe for humans. In some cases, the QD toxicity was not detected in mice because the NPs were neutralized by the liver and accumulated in it [135]; in other cases, QDs coated with phospholipid micelles exhibited reduced toxicity owing to the shell [129]. Despite the extensive in vivo studies on QD toxicity, their use in biomedicine remains an open question. One of the main reasons is that all the delayed effects of QDs cannot be monitored in experimental animals, because their lifespan is as short as a few years, which is insufficient for complete elimination or degradation of NPs.
Conclusions
The potential toxicity of NPs is the main problem of their use in medicine. Therefore, not only positive results of the use of NPs, but also the possible unpredictable negative consequences of their action on the human body, should be scrutinized. The toxicity of NPs is related to their distribution in the bloodstream and lymph stream and their capacities for penetrating into almost all cells, tissues, and organs and interacting with various macromolecules and altering their structure, thereby interfering with intracellular processes and the functioning of whole organs. The NP toxicity strongly depends on their physical and chemical properties, such as the shape, size, electric charge, and chemical compositions of the core and shell. Many types of NPs are not recognized by the protective systems of cells and the body, which decreases the rate of their degradation and may lead to considerable accumulation of NPs in organs and tissues, even to highly toxic and lethal concentrations. However, a number of approaches to designing NPs with a decreased toxicity compared to the traditional NPs are already available. Advanced methods for studying the NP toxicity make it possible to analyze different pathways and mechanisms of toxicity at the molecular level, as well as reliably predict the possible negative effect at the body level.
Thus, it is obvious that designing NPs that have small or no negative effects is impossible unless all qualitative and quantitative physical and chemical properties of NPs are systematically taken into consideration and a relevant experimental model for estimating their influence on biological systems is available.
缩写
- FDA:
-
Food and Drug Administration
- IL-1β:
-
Interleukin-1-beta
- MRT:
-
Magnetic resonance tomography
- NP:
-
Nanoparticle
- QD:
-
量子点
- ROS:
-
Reactive oxygen species
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- TNFα:
-
Tumor necrosis factor alpha
纳米材料


