脂质体纳米医学:药物递送在癌症治疗中的应用
摘要
癌症是一种快速且无法控制的细胞生长导致并发症和组织功能障碍的疾病,癌症的患病率日益增加,这是科学家和医生们严重而紧张的担忧之一。如今,癌症的诊断,尤其是其有效的治疗,被认为是上个世纪健康和医学面临的最大挑战之一。尽管在药物发现和递送方面取得了重大进展,但它们的许多副作用和特异性和敏感性不足,通常会对健康组织和器官造成损害,成为使用它们的巨大障碍。这些治疗剂给药的持续时间和数量的限制也具有挑战性。另一方面,对典型的癌症治疗方法(如化疗和放疗)产生耐药性的肿瘤细胞的出现凸显了对抗肿瘤药物特性的创新、改进和开发的强烈需求。脂质体因其能够储存具有不同物理和化学特性的药物而被认为是纳米医学中药物递送和癌症治疗的合适候选者。此外,通过结合各种聚合物、配体和分子进行化学修饰的脂质体结构的高度灵活性和潜力是脂质体的重要优势,不仅可以增强其药理价值,还可以提高抗癌药物的有效性。脂质体可以提高这些抗恶性细胞药物在体内的敏感性、特异性和耐久性,并为纳米药物的应用提供了显着的好处。我们回顾了脂质体的发现和开发,重点是它们在治疗各种癌症和疾病方面的临床应用。还考虑和讨论了如何改善脂质体药物的特性以及它们在癌症治疗中的机遇和挑战。
图形摘要
<图片>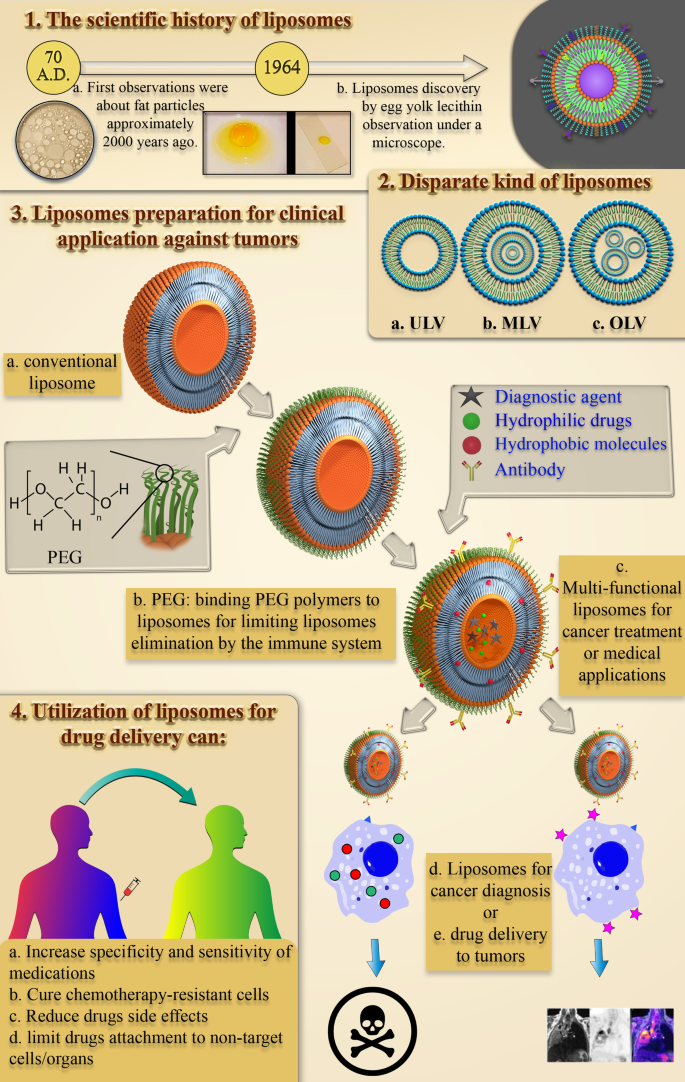
介绍
癌症是一种身体健康细胞脱离正常状态并不受控制地分裂的疾病,被公认为本世纪医学上的一大挑战。这种并发症是由环境致癌物或基因突变的积累引起的[1],被认为是本世纪的一大医学挑战。每年有数百万人死于癌症,而且新发患者的数量和死亡率不断增加[2]。根据世界卫生组织(WHO)的报告,癌症是 2018 年第二大死亡原因,据估计,当年约有 960 万人死于各种癌症。 2018 年,每 6 人中就有 1 人死于癌症。大约 70% 的癌症死亡发生在发展中国家和低收入国家。但发达国家癌症的发病率和死亡率也应考虑[3]。
已知使用抗肿瘤药物的化疗是癌症的重要治疗方法 [4]。由于缺乏适当的敏感性和特异性,使用游离药物的化疗受到限制。因此,这种限制由于副作用而阻碍了准确治疗,并抑制了足够的抗肿瘤作用[5]。化学免疫疗法是一种伴随联合治疗,也被认为是一种有效且有前景的癌症治疗方法,可明确治疗对常规药物具有抗性的肿瘤细胞。近年来,各种常规和先进的治疗方法被发现并应用于治疗癌症。例如,为了减少常规抗癌药物的副作用,特别是化疗药物、各种纳米药物 [6],包括病毒纳米粒子 (VNP) [7, 8]、量子点 [9]、聚合物纳米材料 [10] 和脂质体[11] 已申请。
在不同的纳米药物中,脂质体作为球形纳米粒子 (NPs) 具有特定的结构。脂质体成分中存在的两个水相和有机相允许截留两种亲水性和疏水性试剂,并为脂质体创造了优于许多纳米载体的显着优势。提高抗肿瘤药物的特异性、生物利用度和生物相容性的方法之一是将它们包裹在不同种类的脂质体中 [5]。在过去的 20 年中,已经做出了重大努力来开发脂质体用于治疗目的。其中一些药物,如 DaunoXome® 和 Caelyx®,已被批准用于一般和临床应用,而其他药物正处于最终生产和批准阶段[12]。
通常,存在不同种类的治疗脂质体,例如免疫脂质体和pH敏感脂质体。免疫脂质体是一大类纳米医疗器械,也称为靶向给药系统(DDS),在研究中显示出显着的抗恶性肿瘤作用[13]。 pH敏感性脂质体也称为一组多态性脂质体,其中的结构和组成分子因pH变化而改变,导致其药物成分的释放[14]。此外,脂质体与其他纳米药物一样,除了用于药物输送系统外,还具有用于组织修复和再生、成像和诊断的潜力。脂质体在各个方面的应用简化了疾病和癌症的识别、管理和治疗[15]。
本文对脂质体的发现和结构、脂质体的各种性质、市场上用于治疗癌症的脂质体药物及其发展进行了总结。最后,将总结出脂质体纳米药物利用的机遇和挑战的报告,将其作为科学家未来研究中需要注意的关键问题,从而消除局限性并加强积极点。
正文
脂质体的科学史:发现和定义
从对水性环境中小脂质颗粒的结构和行为的早期研究到第一个美国 FDA 批准的基于脂质的药物递送纳米颗粒,大约花了 1950 年的时间。研究水中脂质和脂肪颗粒行为的过程始于大约 2000 年前 Pliny the Elder 的首次观察 [16]。十七世纪后期,安东尼·范胡克对细胞的发现提出了许多关于细胞结构的问题[17]。然后,Gorter 和 Grendel 发现细胞膜中存在磷脂双分子层 [18]。随后,Singer 和 Nicolson 后来描述了双层镶嵌膜模型来解释细胞膜磷脂的行为[19]。这些科学观察和假设引起了其他科学家对脂肪衍生 NPs 的关注。 1960 年代,在 Babraham 研究所 [20, 21] 研究脂质,尤其是磷脂对血液凝固过程的影响的 Alec D. Bangham 意外地观察到了第一个脂质体,并惊讶地发现在血液中形成了自发的球形颗粒。水[22]。后来,Alec Bangham实验室的访客Gerald Weissmann知道Bangham的研究结果,将Alec观察到的近晶中间相称为“脂质体”而不是“banghosomes”,并因此获得诺贝尔奖[22]。图 1 总结了脂质体发现的科学历史。
<图片>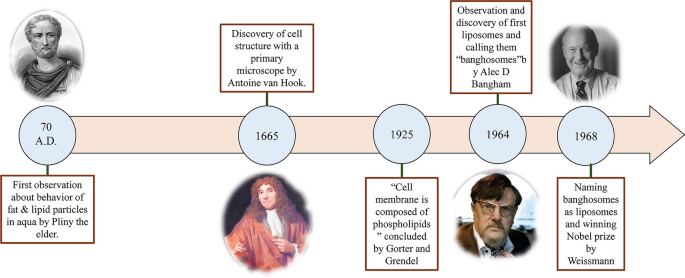
导致发现脂质体的观察图。研究水中脂质和脂肪颗粒行为的历史和科学趋势以及导致脂质体发现的观察结果,以及参与该事件的科学家的图像,Pliny the Elder [23]、Anthonie Van Hook [24]、Alec D. Bangham [25] 和 Gerald Weissmann [26],从左到右
当今称为脂质体纳米颗粒的结构有哪些?
有大量的努力来定义脂质体 NP 并合理地发现它们的特性。目前,脂质体被定义为由脂质双层膜和亲水核组成的自发形成的球形片段。
脂质体的大小从 10 纳米到 2500 纳米(或 2.5 微米)不等 [15]。然而,大多数用于药物递送的脂质体的大小通常约为 50 至 450 nm。当然,更大尺寸的脂质体也可用于医学应用 [27]。此外,脂质体主要由磷脂组成。磷脂是一种脂质,有趣的是类似于甘油三酯。在磷脂的结构中,有一个亲水极和两条疏水链。因此,磷脂被认为是两亲性分子。
磷脂的脂质体膜主要包括磷脂酰胆碱(PC)、鞘磷脂(SM)、磷脂酰丝氨酸(PS)和磷脂酰乙醇胺(PE),它们是两亲性的,在水中极易形成特殊结构[28]。这种现象的物理原因是磷脂中同时存在一个亲水头(磷酸盐分子)和两个疏水尾(脂肪酸)。磷酸基团与 H2O 极性分子相互作用,而疏水尾部从水分子中逸出并相互相互作用 [29]。在这种情况下,非极性链彼此相对放置并形成双层,在它们之间形成亲油空间。因此,脂质体的这种亲脂部分结构可用于储存疏水剂和材料。此外,磷脂的亲水部分然后通过分子力(如氢键、范德华力等)指向水分子,它们出现在它们之间。这些导致在脂质体内部形成亲水区域。卵磷脂分子作为一种天然磷脂,在蛋黄中含量丰富,能够在水中和脂质体的各个区域形成脂质体,其结构如图2所示。
<图片>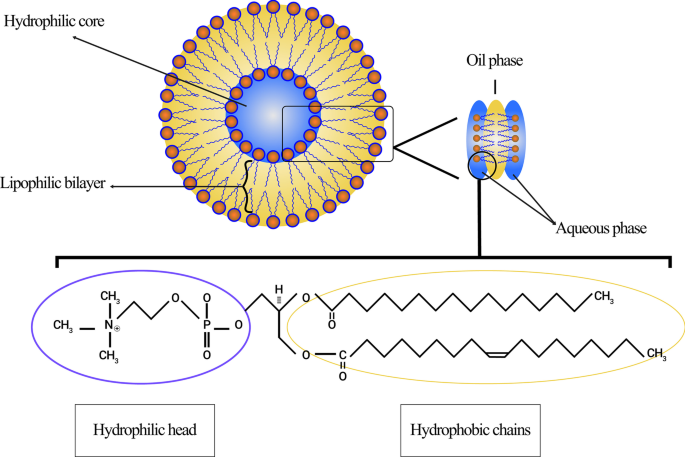
来源于卵磷脂的脂质体示意图。展示了脂质体的不同区域,包括亲水核心和疏水双层。明确了卵磷脂分子的结构、亲水极和疏水链
同样,脂质体在水中溶解后的球形结构取决于分子的类型、水性介质的温度、摩尔浓度以及其他物质(如离子)的存在,决定了其最终形状 [30]。脂质体的主要物理和化学属性是其组成脂质的净特性,尤其是磷脂和构成它的其他分子。这些特性包括渗透性、表面电荷密度和整体尺寸[31]。
不同类型的脂质体分类
自脂质体发现以来,这些结构一直被用作生物学、生物物理、生化或药物研究的重要组成部分。
今天,脂质体可以根据它们的大小、磷脂双分子层的数量、合成程序和制备机制进行分类。就大小而言,脂质体可分为三组:小型、中型和大型。考虑到膜层的数量,它们可以是单层囊泡 (ULV)、寡层囊泡 (OLV) 和多层囊泡 (MLV)。在这方面,ULV 是由一个约 50 至 250 nm 的磷脂双层组成的脂质体,而 MLV 则大得多,约为 0.5–1.5 µm,并包括几个磷脂双层膜 [32]。不同的综合方法导致这两组之间存在边际。在应用方面,ULVs内部也有很大的亲水环境,适合包封亲水性药物。小型单层囊泡 (SUV) 与 ULV 一样,由一个磷脂双层组成,但就尺寸而言,它们的尺寸小于 100 nm [33, 34]。从形态学的角度来看,OLV 是由两到五个大小相同或不同的囊泡组成的脂质体。在 OLVs 的结构中,囊泡都被包裹在一个大的磷脂双层中,而不是彼此内部。 OLV 通常约为 0.1–1 µm [33,34,35]。与 ULV 相比,MLV 不是输送亲水性物质的理想选择。 MLV 主要用于输送疏水剂 [36]。不同类型的脂质体如图 3 所示。
<图片>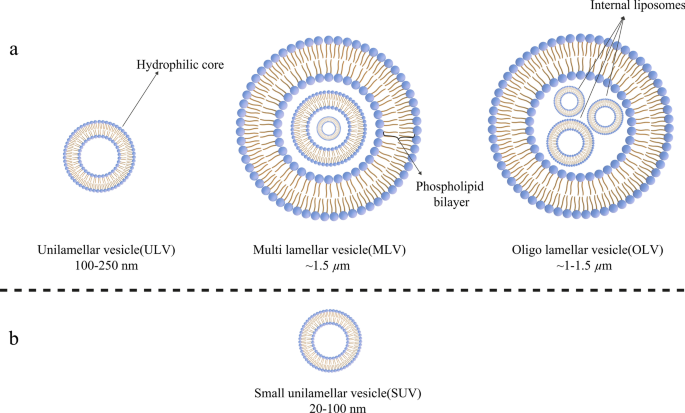
根据不同标准对脂质体进行分类:a 脂质体按大小分为三类; b 小单层囊泡(SUV)结构,作为单层囊泡(ULVs)的成员,具有明显的小尺寸
脂质体制备方法及其新一代开发
与作为硬纳米颗粒的金纳米颗粒相比,脂质体是软纳米颗粒 [37],可以通过不同的方法合成。例如,MLV 和 ULV 具有不同的制备机制。在大多数这些方法中,使用特定溶剂(例如氯仿或甲醇等)来溶解旨在在圆底烧瓶 (RBF) 中形成脂质体膜(具有所需摩尔比)的脂质。例如,握手是合成 MLV [38] 的主要程序。在这个过程中,也被认为是脂质膜水合,将脂质添加到有机溶剂中。然后,通过旋转装置蒸发溶剂,固体产物被脂解。最终,脂质体是通过水合和挤出方法合成的 [39]。脂质体合成的其他方法包括超声处理、反相蒸发、法式压力池、冷冻干燥和膜挤出 [38, 40]。
此外,脂质体也可以根据它们的发现和随着时间的发展而分为不同的类别。第一代脂质体通常称为常规或经典脂质体。使用常规脂质体作为治疗性纳米颗粒时观察到的问题在体内很快就被发现。早期研究的一个问题是药物包埋到脂质体中的局限性。换句话说,许多药物无法储存在第一代脂质体中 [41]。随着对脂质体的结构和性质(例如稳定性、治疗效果和临床应用的可能性)的极大渴望,这些挑战导致通过改变成分脂质、表面电荷、净重、和总体积 [42]。准确地说,第二代脂质体主要是通过在常规脂质体中添加一些亲水性聚合物来合成,以提高其在体液中的保质期,使其成为药物递送系统的合适候选者。这种脂质体可分为两类:非特异性长循环脂质体或配体靶向长循环脂质体[43]。
古菌体作为新一代脂质体,由古菌膜脂和合成磷脂类似物组成。在过去的十年中,已经做出了广泛和相当大的努力来研究古细菌在药物和疫苗输送中的潜力。古菌类脂质的结构核是二醚或四醚分子,具有饱和的植烷酰基链,含有约 20 至 40 个碳原子。这些碳链与在古菌醇或caldarchaeol 中发现的主链甘油的sn-2,3 碳的醚键相连。如上所述,这些颗粒还可以大量用于治疗肿瘤并发症、过敏和感染的药物递送,以及疫苗接种 [44]。
评估脂质体的生物材料特性和理化性质
如前所述,尽管医学科学取得了广泛的进步,但由于治疗药物和方法效率低下,一些疾病特别是癌症的治疗仍然面临着巨大的挑战。由于抗癌药物的治疗窗口狭窄,调整注射药物剂量以影响肿瘤是一个紧张的问题。换句话说,治疗剂量与毒性剂量之间的微小差距,以及不适当的敏感性和特异性,对先进的治疗程序产生了巨大的需求[42]。
此外,利用纳米材料将药物输送到组织最近引起了人们的注意。生物相容性和生物降解性是生物材料特性的两个基本特征,可用于纳米材料在递送系统中的利用。需要生物相容性来抑制治疗性 NPs 对身体组织和系统的破坏,而生物降解性对于将 NPs 分解成无毒化合物并简单地从器官中去除是紧迫的 [15]。在脂质体检测之后,科学家们开始将它们作为纳米材料用于药物递送。如前所述,脂质体具有治疗目的所需的两种生物材料特性:生物相容性和生物降解性 [36]。此外,脂质体 NPs 具有其他特性,使其适用于此目的。例如,由于脂质体的特殊结构,亲水性(水溶性)和疏水性(脂溶性)药物都可以封装在其中。此外,脂质体中磷脂双层膜的存在保护储存在脂质体中的试剂免受各种现象和损害,例如酶降解、免疫结构导致的生物失活和体内化学变化。这一点有两个重要的优点:首先,包裹在脂质体中的分子结构在到达目标组织之前被保留下来,没有对其进行任何修改,其次,其他健康和非目标组织受到保护,不会暴露于药物由于脂质体膜,不受这些药物的影响 [42]。脂质体还可用于传递遗传物质,例如 DNA、RNA 等,以及基因治疗目的。用于此目的的脂质体可以由阳离子、阴离子、中性脂质和磷脂或它们的混合物组成 [45]。一些诊断和显像剂,例如碳点,可以与脂质体组合或单独用于癌症检测和成像 [46]。尽管碳点部分被批准用于临床应用并在研究中得到利用,但细胞毒性仍然是其广泛应用的一个具有挑战性的障碍 [47]。脂质体药物纳米粒的一般结构见图4。
<图片>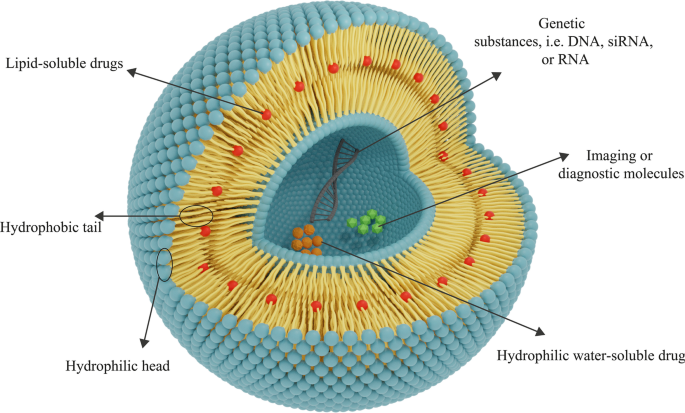
脂质体的一般结构由磷脂层组成。根据药物的亲水性-疏水性,将确定适合其递送的脂质体类型。亲水性药物被包裹在中央亲水核中,疏水性药物被放置在亲脂区。脂质体也可用于基因的传递
脂质体中存在的成膜磷脂是无毒化合物,可以合成多种尺寸。脂质体的物理化学特性取决于它们的成分。因此,通过添加某些化合物,如胆固醇、聚乙二醇 (PEG) 等,可以合成具有所需特性的脂质体。此外,脂质体的膜对大分子是不可渗透的,这有助于更好地将物质保留在脂质体内 [48] ]。脂质体的所有上述特性使其成为一种合适的纳米材料,可用于递送治疗各种疾病,尤其是癌症的治疗剂。
Gregory Gregoriadis 作为该领域的先驱研究人员之一,提出了将脂质体用于药物递送系统的假设,并指出药物化合物可以被包裹在脂质体中 [49]。已经报道了脂质体的适当生物材料和理化特性。例如,一项对用于动物模型的含有抗肿瘤药物阿糖胞苷的脂质体的调查显示,患有 L1210 白血病的小鼠的寿命显着增加 [50]。通过应用脂质体,可以将足够剂量的药物活性形式以受保护的方式递送至靶位[42]。
脂质体纳米粒治疗用途的特异性和敏感性的增强
正如前面提到的,使用各种分子和聚合物可以改变脂质体的结构和膜,通过这种方式,可以向脂质体添加新的功能或改变它们的性质 [51]。由于脂质体的高清除率,延长脂质体在血液中的循环并增加它们通过 EPR 效应在特定肿瘤组织或病理部位积累的能力是首先应考虑的重要特征。 PEG 分子通过化学缀合与脂质体膜的缀合已被顺序用于将这种功能添加到脂质体中 [52]。乙二醇聚合物在增加脂质体半衰期方面的重要性和作用,尤其是体液(如血液)中的脂质体治疗性纳米粒,早在 20 年前就已表达[53]。
Abuchowski 和 McCoy 首次尝试通过将 PEG 结合到脂质体的结构来延长脂质体在血流中的半衰期。因此,他们的努力通常会增加脂质体的循环时间及其在血流中的半衰期 [54]。几个月后,其他研究人员研究了降低单核吞噬系统细胞 (MPS) 对脂质体的高速清除的可能性。通过将 PEG 连接到脂质体的表面分子 [53],预计脂质体在血液中的循环会得到改善。这个领域有很多文章。此外,与常规脂质体不同,PEG 包被的脂质体表现出与剂量无关的药效学特性 [55]。在各种聚合物中,PEG 分子是可以附着在脂质体表面以延长其体内保质期的聚合物之一。其他聚合物也可用于此目的[56]。图 5 展示了 PEG 聚合物分子如何保护脂质体免受抗体侵害并延长其在血流中的寿命。
<图片>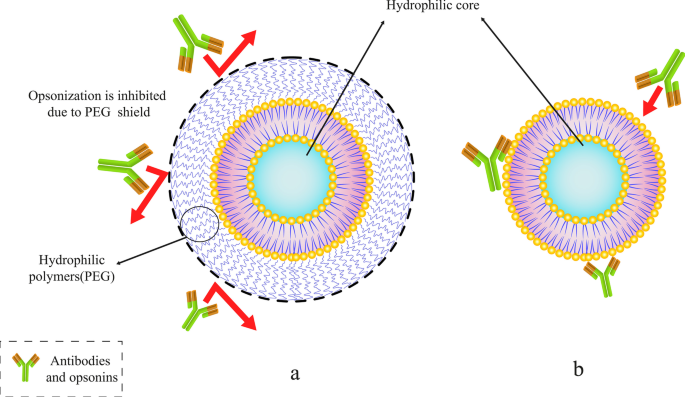
将特定聚合物(如聚乙二醇 (PEG))与脂质体结合。 一 带有 PEG 聚合物分子的 PEG 化脂质体屏蔽。 b 常规脂质体被抗体和调理素捕获
如上所述,值得注意的是,除了 PEG 之外,还可以利用其他分子来延长脂质体的循环。此外,聚恶唑啉聚合物是用于修饰脂质体膜以提高其半衰期的物质之一。在这方面,伍德等人。是第一个应用聚[2-乙基-2-恶唑啉](PEOZ)合成隐形脂质体的小组。他们的结果证明了肝脾细胞注射到大鼠体内的聚[2-乙基-2-恶唑基化] PETOXylated 脂质体的消除和摄取减少 [57]。他们的研究结果表明,聚[2-乙基-2-恶唑啉]和聚[2-甲基-2-恶唑啉](PMOZ)等其他聚合物的共轭作用在增加半衰期和延长脂质体在体内的循环。他们还比较了 PEG-、PEOZ-和 PMOZ-缀合的脂质体在各种器官和系统中的生物分布。结果还表明,所有这些脂质体在血液和脾脏中的生物分布几乎相同,但在肝脏中,PMOZ的分布远低于其他[57]。
疼痛等。将葡聚糖分子结合到 ULV 的表面。他们的结果表明,与常规脂质体相比,葡聚糖偶联脂质体具有更长的循环时间和更低的肝脏和脾脏吸收和摄取。这一结果证明,葡聚糖分子除了可以延长脂质体在体内的保质期外,还可以用于增加脂质体的稳定性和调节药物释放速率[58]。
第二个需要注意的问题是脂质体的流动性和稳定性。其他脂质,包括胆固醇,可用于脂质体框架中。胆固醇有时可能会替代磷脂双层中的某些化合物,以增强脂质体的某些特性。尽管如此,已经证明改变脂质体双层的含量并用某些化合物,尤其是胆固醇代替一些磷脂分子,可以降低脂质体的流动性[59]。此外,脂质体膜中胆固醇的存在增加了其结构的稳定性(体内和体外实验)。它还降低了渗透性和夹带物质泄漏的可能性。胆固醇是一种疏水类固醇,当存在于脂质体膜中时,它与磷脂双层之间的疏水链相互作用以稳定其结构。当在体内临床利用脂质体时,胆固醇的这种作用很重要,因为它可以防止脂质体在体内转化为高密度脂蛋白 (HDL) 和低密度脂蛋白 (LDL)。此外,血液和细胞内液中存在的脂质结构会对脂质体产生影响。低密度脂蛋白和高密度脂蛋白等脂蛋白会影响注射的脂质体并导致脂质转移和膜重排。它们还大大降低了含药物脂质体 NPs 的稳定性 [12]。值得注意的是,其他材料,例如 DNA 和用于治疗应用的脂质体膜中的其他分子,必须锚定在膜中的胆固醇上。向脂质体膜中添加各种物质是在脂质体中创造积极特征的一种方式[42]。
应考虑的第三个基本特征是脂质体对准确识别和与靶细胞特异性结合的敏感性和特异性。通过结合化合物,如单克隆抗体、Fab 片段和其他结合分子,如转铁蛋白和叶酸,可以增强脂质体的特异性,导致与肿瘤细胞的特异性结合 [60]。此外,之前已经研究过提高药物纳米载体,特别是脂质体的特异性和敏感性。例如,Mohammad J. Akbar 等人。研究了肽-PEG-脂质偶联脂质体治疗小细胞肺癌(SCLC)。他们的结果表明,将胃泌素释放肽受体 (GRPR) 拮抗剂肽与脂质体结合可以增加这些脂质体在 GRPR 表达细胞中的特异性和积累。他们还声称,这些附着在肽上的脂质体可用于治疗肺癌细胞,因为它们中的 GRPR 表达基因上调 [61]。
最终,药物和药物因其适当的特性而被添加到聚乙二醇化脂质体中,现在,这些脂质体结构已被建立用于工业临床应用[62]。早期研究中也使用抗体来增加脂质体与靶细胞结合的能力 [63]。在这种情况下,脂质体通过受体介导的内吞作用进入细胞[64]。同时,已经开发了多种方法来将抗体与脂质体结合 [65]。对抗体偶联脂质体的研究已经证明,抗癌药物对培养的肿瘤细胞的毒性随着抗体与脂质体表面的偶联而增加 [66]。当将抗体应用于 PEG 脂质体的表面时,附着在其靶标受体上的抗体残留被 PEG 聚合物掩盖,尤其是当附着在 PEG 上的侧链很长时 [67]。因此,PEG和抗体同时用于脂质体药物治疗及其弊端应引起科学家们的重视。
脂质体治疗应用的第四个重要因素是包裹在脂质体中的药物释放的练习曲。脂质体中受损伤组织异常状态影响的药物的解脱是脂质体临床给药的关键问题之一。此外,在脂质体表面使用温度敏感化合物、pH 值或特定代谢物,可以与靶细胞的组织和膜表面结合,是一种精确释放这些药物的方法。利用该方法可以使脂质体对靶细胞膜表面产生特异性作用,同时释放出其中的药物成分[68]。
The releasing rapidity of compounds entrapped in liposomal NPs is the fifth substantial criterion for adjusting the dose of drugs available at the target site. One of the essential objects that should be considered for the proper usage of all kinds of drug delivery systems, including liposomes, is the releasing rate of drugs and regulation. With regard to liposomal drug delivery systems and NPs, it is worth mentioning that the encapsulated substances in the liposomes are not biologically available and can only be bioavailable while it is released from the initial state. Therefore, drug-containing liposomes can provide the ability to increase the concentration of bioavailable drugs for cancerous tissues and to improve the quality of treatment and therapeutic efficacy can be achieved on condition that the rate of drug release from the liposome is adjusted [69]. Furthermore, it has been proven that changing the liposome bilayer content and replacing some phospholipids with certain compounds, especially steroid molecules like cholesterol, can decrease the permeability and unintended leakage of the compounds stored in them [70]. Consequently, this advantage can be exploited to adjust the release rate of the encapsulated compound. Once released, the drugs must penetrate sufficiently into the cell and make the necessary physiological-biochemical changes to exert their impact.
As it is mentioned earlier, various compounds, including aptamers, can be conjugated to liposomes. In this regard, Mohammad Mashreghi et al. applied anti-epithelial cell adhesion molecule (anti-EpCAM) as an aptamer to functionalize Caelyx® liposomes. Their experiment outcomes determined that functionalization of Caelyx® with this aptamer could enhance the merits of this liposomal drug and made it a viable option for cancer treatment [71]. Figure 6 shows the structure of different types of liposomes that are used in vitro or for clinically scientific purposes schematically.
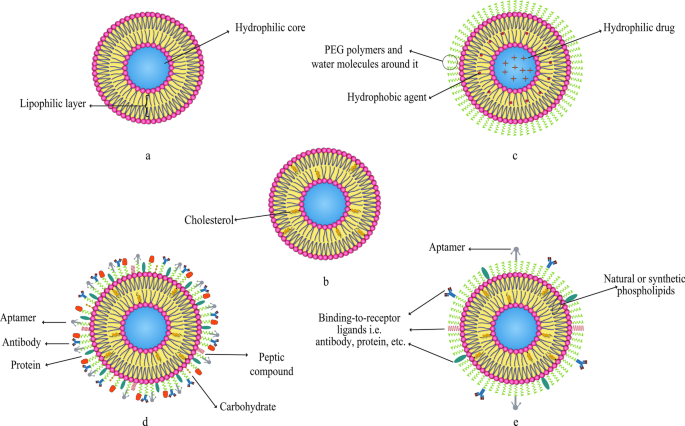
Various kinds of liposomes. 一 Conventional liposome; b cholesterol-conjugated liposome; c PEGylated or stealth liposome; d ligand-targeted liposome; e multi-functional liposome
The passage of drugs through lysosomes to enter cells (which have low pH and many degrading enzymes) is the sixth most important factor for the practical application of conjugated medicines to liposomes. To protect therapeutic agents from unwanted conversions in extracellular and intracellular space, cell-penetrating peptides are attached to the liposome surface [72].
On Liposomal Drugs Pharmacology:Pharmacokinetics and Pharmacodynamics
The assessment of pharmacological attributes, as an essential part of medicine and pharmaceutical science, is required not only to gain a better understanding of liposomes pros and cons as drug carriers but also to confirm and evaluate them in clinical trials. The pharmacological properties of liposomal drugs and their interactions with the body can be examined in two various aspects:pharmacokinetic (the effect of the body on therapeutic compounds) and pharmacodynamics (how medications act and impact the body and cellular pathways) [73]. In general, the utilization of liposomes for drug delivery in cancer treatment or other disorders requires the elevation of these agents' effectiveness on the one hand, and reducing their toxicity toward normal tissues on the other hand. Subjects such as the proper administration route of NP-based drugs, their circulation in the bloodstream and half-life, their biological distribution in tissues, and their cellular metabolism, as well as their elimination, metabolization and clearance, have been studied in the field of pharmacokinetics [74]. The pharmacokinetics of liposomes primarily study the bioavailability of liposome-conjugated drugs in various body fluids and tissues. Indeed, the study of chemical decomposition and biological excretion, and liposome uptake and purification are also considered in pharmacokinetics. The results of studies on pharmacological advantages of using liposomal drugs (regardless of the type of liposomes applied in the DDS) instead of free drugs showed that:
Primarily, liposome can modify the drug release profile to a sustained release, and consequently, reduce the requirement for constant injection. Secondly, it can extend the presence of the drug in the bloodstream and body fluids, and as a consequence, increase its half-life. Thirdly, it has the potential to lead to better bio-distribution in cancerous tissues while reducing drug influences on healthy tissues due to limited particle size to cross the Endothelium of healthy capillaries. Ultimately, it reduces drug metabolism and inactivation in plasma before reaching the target tissue, in addition to its positive effect on the clearance of drug metabolites [75, 76].
However, some changes are required in the pharmacokinetics of liposomes to increase their solubility, specificity, and sensitivity. These modifications enable them to overcome chemotherapy-resistant cells, enhance the efficacy and half-life. Moreover, their toxicity or unintended metabolic compounds product as a result of their metabolization should be decreased by these modifications [77].
After consuming liposomal drugs administration, they enter the body and circulate in the bloodstream with a specific half-life. Their size and formative composition determine the half-life of liposomal medications. Moreover, rapid clearance of liposomal drugs from the body can reduce their duration of action and therapeutic index. As aforesaid, appending hydrophilic polymers such as PEG to liposomes is able to decrease their clearance rate and solve this challenge [78]. Also, it is possible to adjust the fluidity and drug-release rate of the liposome membrane by adding cholesterol molecules.
The application of liposomes for drug delivery may lead to some changes in drug pharmacokinetics [79]. The ability of liposomes to change the pharmacokinetic properties of the various drugs and medications is one of their significant benefits in drug delivery systems [80]. Concerning the process of liposome clearance and elimination, it is obvious that liposomal structures are affected by plasma proteins after being administered. For instance, after injection of liposomal nanoparticles opsonins are adsorbed on the surface of the liposomes. Opsonins are plasma protiens which mostly include immunoglobulins and fibronectin [42]. Opsonins presence on the surface of liposomes will result in their elimination by MPS, as one of the significant elimination section of various drugs from blood and body fluids. They also clear liposomes through the attachment of some receptors such as complement C3b and Fc to opsonins-liposomes complex [81]. Various tissues and cells such as liver kupffer cells, macrophages present in the spleen, bone marrow, and lymph nodes are involved in the clearance of liposomal NPs [82].
According to the International Union of Pure and Applied Chemistry (IUPAC) definition, pharmacodynamics refers to the study of the pharmacological impact of compounds on living systems and the biochemical and physiological consequences of these effects [83]. The increased elusion to identify therapeutic agents when encapsulated in liposomes has been recognized as one of the pharmacodynamical benefits of liposomes utilization [84].
Furthermore, the physicochemical characteristics have significant influences on the pharmacology of liposomal drugs. The particle size, electrical charge of membrane, and the composition of membrane lipids are some of these physicochemical properties that can affect the pharmacokinetics and pharmacodynamics of the agents. Firstly, there is a direct relationship between the particle size of nanoparticles, including liposomes, and their clearance rate. By increasing the size of NPs, their elimination rate by the immune system and MPS cells will also enhance [85]. Secondly, it is worth mentioning that the net charge of liposome membranes is a consequence of the electrical charge of phospholipids and their other constituting particles that made them up. As a result, a rise in the membrane charge is associated with enhanced clearance rates of these agents [86]. The composition of membrane lipids and other structural features (such as hydrophilic core radius) also remarkably affect the pharmacokinetics of liposomal drugs [87].
More importantly, it has been hypothesized that different types of liposomes exhibit distinct drug kinetics/dynamics depending on their various structures. The drug release rate also rests on the number of phospholipid bilayers and the content of loaded drug compounds. It is also contingent upon the hydrodynamic diameter, total volume, and other pharmacokinetic properties as well [88].
Administration Route of Liposomal Drugs
Like many different drugs, NP-based liposomal medicines can be administered from a wide variety of routes. In other words, oral consumption [89] and distinct injection methods such as intravenous (I.V.) administration and various local injections are among the common administration routes of liposomal drugs [90]. The usage of nanoparticles, including liposomes, for drug delivery via oral administration has been highlighted as an effective strategy since the nanoparticles increase the bioavailability of medicines, improve their interaction with cells, and prevent any modifications in the molecular structure of the drug due to enzymes and gastric juices in the gastrointestinal tract. Moreover, they have the ability not only to enhance the release of remedial molecules into the mucosal and epidermal layer but also to protect drugs from unwanted changes during the first pass effect [89]. Intravenous injection is used as the primary administration route for many liposomal drugs approved by the FDA or other authorities [42]. On the other hand, subcutaneous (S.C.), intradermal (I.D.), intraperitoneal (I.P.), and intramuscular (I.M.), classified under the title of the local injection, are also utilized for administration of liposomal drugs [90,91,92].
Liposomal Drugs Fate In Vivo and Their Targeting Mechanism of Action
Following administration of the liposomal drugs, they reach the pathological lesions at the target site through the bloodstream and accumulate there. The mechanism of action of liposomal drugs on tumors starts with their accumulation at the target site, uptake of them by tumor cells, and the release of free drugs [93]. Subsequent to entering the body, liposomal drugs reach the tumors through various targeting mechanisms of action and then interact with cells in different ways [94]. In general, tumor-targeting mechanisms are divided into two categories:passive and active targeting. Passive targeting refers to the mechanisms in which liposomes are spontaneously accumulated at the tumor site and interact with target cells without the presence of a specific ligand [95]. The effect of enhanced permeability and retention (EPR) has been suggested as the most critical passive targeting mechanism. To be precise, the spontaneous accumulation of therapeutic NPs and liposomal drugs at the tumor site is called the EPR effect [96]. This phenomenon can be assigned to the leaky nature of tumor tissue vessels, unlike normal tissue capillaries, which makes them permeable to molecules and NPs. Consequently, This ultimately leads to the accumulation of drug compounds in these tissues and the effect of EPR [97]. The ultimate fate of the drug in the intracellular fluid and cytoplasm of tumor cells depends on several factors such as release mechanism, nanocarrier constituents, and molecule structure [98]. In healthy tissue, the number and the shape of capillaries are proportionate and normal, respectively. However, in cancerous organs, unlike healthy tissue, the number and the structure of capillaries are higher and deformed, respectively, because of the angiogenesis process. Moreover, the tumor capillaries structure is destroyed, and the endothelial phalanx cells are diminished. As a result, the volume of plasma fluid leaking into the intercellular space will be enhanced. In healthy tissue, however, capillary phalanx cells retain cellular tight adhesions, preventing NPs, small molecules, and liposomal drugs from seeping into the intercellular space [99]. The EPR effect in cancerous capillaries and their difference with normal and healthy tissue vessels are illustrated in Fig. 7.
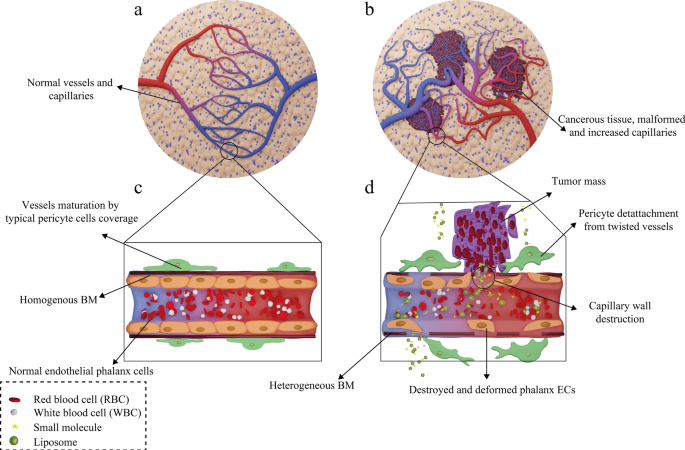
Mechanism of action of the drug-containing liposomes on tumor cells via EPR effect. 一 Healthy tissue and its normal capillaries; b cancerous tissue with increased-deformed vessels; c structure of normal and healthy vessel; d destructions and deformed capillary in tumor tissue
On the other hand, active targeting has attracted considerable attention as one of the targeting mechanisms of action owing to its appropriate effectiveness and high specificity. Active targeting includes various types and is also generally aimed to reduce the off-target impacts of liposomal NPs on healthy cells and non-target tissues [95]. In this method, molecules such as monoclonal antibodies, small molecules, signal peptides, vitamins, particular carbohydrates, glycolipids, or aptamers are generally utilized for surface modification of liposomes [100, 101]. Moreover, active targeting can be split into various subtypes according to diverse features. For instance, it can be classified into two general categories:
- 1.
Targeting tumor cell and cancer tissue receptors:This method relies on conjugating specific molecules to the membrane surface of liposomes, making them able to bind to special or overexpressed receptors on cancer cells [102]. In cancer cells, upregulation of different genes causes an increase in the expression of specific cell surface receptors in response to enhanced metabolic demands for rapid cell proliferation [103]. In active targeting, particular molecular modifications can be applied for targeting specifically the overexpressed surface receptors of cancer cells, such as folate receptor (FR), transferrin receptor (TfR), or Epidermal growth factor receptor (EGFR) [95]. In this regard, the role of folate receptors in cancer cells is to increase folic acid uptake [104], whereas transferrin receptors bind to transferrin (as a free molecule with 80 kDa weight in serum) and cause endocytosis of this monomeric glycoprotein to occur [105]. Moreover, EGFR receptors are a class of tyrosine kinases involved in cellular processes such as tissue differentiation and repair. The expression of this receptor in cancer cells is significantly increased due to its involvement in processes such as angiogenesis, cell proliferation, and metastasis [106].
- 2.
Utilizing tumor microenvironment as the target:In this method, changes in the surface of liposomes are exploited to enable them to target signal peptides or other receptors in the microenvironment of cancer cells. In other words, this active targeting mechanism can inhibit the growth of tumor cells and metastasis, prevent genotypic and phenotypic variations in neovascular endothelial cells, and control drug resistance [107]. Furthermore, some receptors in the tumor microenvironment, such as Vascular endothelial growth factor (VEGF), Vascular cell adhesion protein (VCAM), matrix metalloproteases, and integrin, are targeted in this mechanism [95].
Cellular Uptake of Therapeutic NPs and the Effect of Liposomal Drugs on Targeted Cells:Actions and Interactions
As it is mentioned earlier, liposomes are able to target tumor cells either passively or actively. After the liposome reaches the cancerous cells and the tumor environment through the targeting mechanism, it can release its therapeutic content and exert its effects by means of various mechanisms. Consequently, lipid composition, the surface charge of the membrane, type of cancer, type of target cells, as well as the presence of specific ligands on the liposome membrane, can influence the cell-liposome interaction [108].
Figure 8 illustrates different types of liposomes interactions with target cells. After being injected into the body, drug-containing liposomes travel to different tissues through blood vessels and eventually reach their target cells based on their surface ligands. These liposomes can bind to cellular receptors via these ligands, which is called specific absorption [42]. Albeit, receptor-free liposomes can also adhere to the target cell surface through molecular attractions, electrostatic forces, and molecular interactions called non-specific absorption. Following liposomes binding to the cell, the therapeutic agent is released into the cytoplasm, and its effects may be produced in different ways. The liposomal nanocarriers can be entirely fused to the plasma membrane of the cell and release the drug. Drug compounds are also able to be released from the liposome into the cell and to enter the cell through micropinocytosis or passive diffusion without the occurrence of fusion. Liposomes may directly interact with the cell or exchange lipid fragments with the cell membrane through protein-mediated processes. At the same time, the drug may act on the cell and exert the therapeutic effects of the liposomal drug. However, some liposomes are capable of entering through endocytosis (specific or nonspecific). In particular, liposomes penetrating the cell via this passage can have various destinies. It is possible for them to combine with lysosomes. In such cases, lysosomal enzymes affect the structure of the drug by reducing the pH of the phagolysosome sac. Ultimately, liposomes release the drug by fusing it to the cell membrane or endocytosis, and after that, medications exert their therapeutic effect [42, 62]. All possible ways for the liposome to penetrate the cell and exert its effect are depicted and compared in Fig. 8.
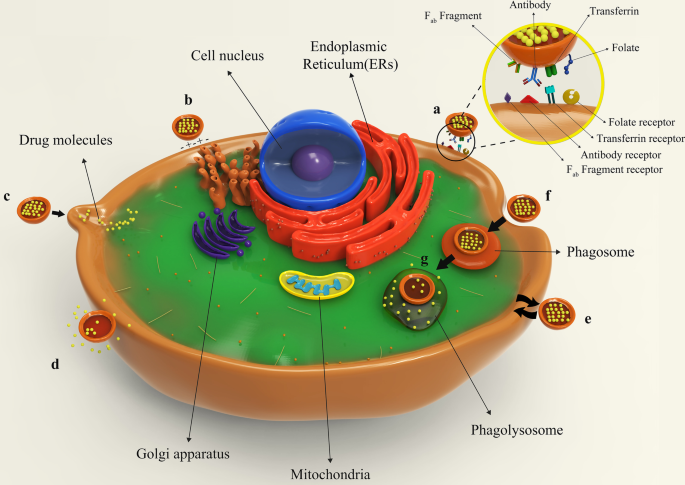
Binding of liposomes to the target cell. 一 Specific attachment via ligand-receptor interaction; b non-specific absorption of liposomes through intramolecular-electrostatic forces; c the attachment and fusion of liposome to the cell membrane and drug release; d liposome arrival to the target cell and drug release without fusion; e exchange lipid fragments between the cell membrane and liposome through protein-mediated processes; f endocytosis of liposome by target cell; g lysosomal digestion of liposome in the cell cytoplasm
On the other hand, NP-based medications can undergo endocytosis, pinocytosis, or phagocytosis by the target cells. Endocytosis is known as the process in which compounds outside the cell space approach the cell membrane and then enter the cell as a vesicle [109]. Pinocytosis, also recognized as fluid endocytosis, occurs when small molecules or suspensions are introduced into a cell through a vesicle by creating an invagination in the cell membrane. Moreover, pinocytosis vastly occurs in human cells to absorb fat droplets. In an immunological study, Yuriko Tanaka et al. reported that liposome-coupled antigens pinocytosis can be performed by antigen processing cells (APC). This report had proved that liposomes can undergo pinocytosis mechanisms [110]. In phagocytosis, particles larger than 0.5 μm are engulfed by immune cells, it may also occur for liposomes (especially for MLVs and liposomes larger than 500 nm). For example, Jitendra N. Verma et al. confirmed the occurrence of phagocytosis on liposomes by a study on the phagocytosis of liposomes with malarial antigens by macrophages [111].
Kaposi's Sarcoma, One Instance of Successful Liposomal Drugs Applications
Kaposi's sarcoma is a progressive multifocal anti-proliferative cancer primarily known as endometrial sarcoma. This cancer is more common in HIV patients whose immune system is weakened. Furthermore, it has been commonly seen in skin tissue and may also involve other tissues. Hence, this disorder is generally referred to as skin mucosal sarcoma [112]. To treat this disease, modified long-circulating liposomes can be helpful. In this regard, liposomes passively target tumor cells. Moreover, the effect of EPR and specific binding increases the concentration of the therapeutic drug in cancer tissues 5 to 11 times higher than normal skin [113]. For this purpose, Doxorubicin is used for the treatment of this disease. Correspondingly, entrapment of the doxorubicin into liposomes (which was PEGylated to prolong its half-life) prevents normal tissues from being exposed to the drug. It also reduces drug uptake by these healthy doxorubicin-sensitive tissues such as the heart [114].
Additionally, the liposomal form of doxorubicin, Doxil, is a type of anthracycline drug which is approved for clinical administration by US-FDA. It is used to treat AIDS-related Kaposi sarcoma and multiple myeloma [115]. Doxil has better therapeutic efficacy and less toxicity than free doxorubicin, which can be attributed to its ability to target tumors indirectly. It is also passive targeting due to leakage of tumor vessels and the EPR effect [116]. Moreover, the Doxil unilamellar liposomes are < 100 nm in size and have been used to treat various cancer types [42]. Analyses have also proved that free doxorubicin concentration is lower than that of Doxil at the target tissue site [117]. In this regard, Ogawara et al. investigated the effect of Doxil (formed by binding doxorubicin to PEG liposomes) on cancer cells in male mice and showed that PEG liposomal doxorubicin or Doxil1 had been effective on both doxorubicin-resistant and doxorubicin-sensitive C26 cell groups [118]. This can highlight the significance of the exploitation of liposomal NPs. Because they can be consumed to overcome the resistance of cancer cells to common chemotherapy agents at low costs without time-consuming research works to discover new clinical therapeutic compounds [119]. The application of nanoparticles, such as liposomes, to deliver doxorubicin to tumor tissues has been widely investigated. Entrapment of ATP-binding cassette transporter superfamily B member 1 (ABCB1) substrate doxorubicin into liposomes can increase drug uptake and enhance its intracellular distribution within cancer cells, especially ABCB1-expressing cancer cells [120]. The simple structure of Doxil is illustrated in Fig. 9.
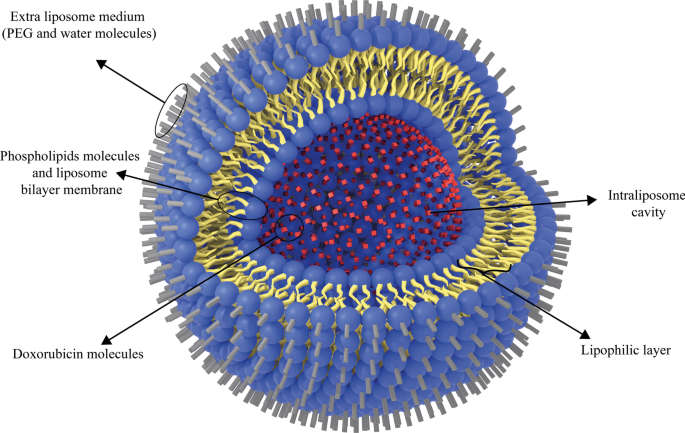
The schematic structure of Doxil drug. Doxorubicin drug molecules are entrapped in the hydrophilic cavity of unilamellar PEGylated liposomes
Furthermore, liposomal nanomaterials can be exploited for the treatment of infectious diseases. Systemic fungal infection is one of the most challenging conditions that is usually treated with amphotericin B, which is highly toxic to kidney cells. For this purpose, the usage of liposome-entrapped amphotericin B can reduce the toxicity of this drug compared to its free form [48]. Unilamellar liposomes have been used to entrap this agent. It has proven that liposomal amphotericin B is more effective than the free drug form [121]. Based on the formulation, these liposomes also alter the bio-distribution of amphotericin B, such as anticancer drugs, which in turn not only arrange the mechanism of action but also increase the effective dosage concentration at the target tissue [122]. AmbiSome, liposomal form amphotericin B, is approved for public administration too. Other approved liposomal drugs, from anti-fungal medications to cancer therapeutic agents, are summarized in Table 1.
Although the application of liposomal NPs to treat cancer has been touted as a viable solution for drug delivery and affecting tumor cells, drug delivery to cancerous tissues in the central nervous system (CNS) has remained a significant challenge. In addition, drug delivery to central nervous system cells faces many turbulences owing to a blood–brain barrier (BBB). However, this problem can be partially solved by developing new methods and using lipid-based compounds [136].
Liposomal Nanoparticles in the Investigational Phase for Therapeutic Purposes
Liposomal siRNA
RNA is a type of genetic molecule with a variety of functions, including translation and transcription processes. The discovery of small-interfering RNA (siRNA) is a significant advance in biology in the last decade [137]. Synthetic siRNAs can be utilized to target oncogenes and their mRNAs. Furthermore, siRNAs can be applied for targeting genes contributing to the carcinogenesis, proliferation, and metastasis of tumor cells or their resistance to standard chemotherapies and radiation [138]. Therefore, it has been considered a modern method for cancer therapy. On the other hand, the nanoparticles used to deliver siRNA must possess properties such as biodegradability, great bio-distribution, low toxicity, etc. All of these features can be offered by liposomes making this popular drug delivery system a promising candidate for this purpose [28]. siRNAs bound to neutral lipid-based NPs are well isolated from these liposomes. They also influence ephrin type-A receptor 2 (EphA2), focal adhesion kinase (FAK), neuropilin-2, Interleukin 8 (IL-8), and TROJAN Mobile Remote Receiving System/erythroblast transformation-specific (TMRRS/ERG), Elongation factor 2 kinase (EF2K) or Bcl-2 pathways. Following the occurrence of this mechanism, a suitable antitumor effect has been observed against ovarian, colon, and breast cancer cells, etc. [139, 140]. Numerous studies have been conducted on siRNA delivery by liposomes, and in most of them, the cationic lipid Dioleoyl-3-trimethylammonium propane (DOTAP) has been widely expended in the structure of liposomes. Due to DOTAP high positive charge, this cationic lipid can be toxic to cells. It can stimulate cellular hemolysis and reduce ultimate biocompatibility as well. This has challenged the application of this lipid in the composition of liposomes applied for siRNA delivery [141].
Liposomal Curcumin Nanoparticles
Curcumin-conjugated liposomes are another instance of liposomal nanoparticle usage. Curcumin is a natural polyphenolic and hydrophilic compound that is abundant in the Curcuma longa plant and can be mainly prepared from turmeric extraction. Nowadays, the anticancer effect of curcumin has been well indicated against many tumor cells, such as breast cancer, liver carcinoma, and prostate cancer, etc. [142]. The primary mechanism of action of curcumin against cancer cells is to interfere with the translation of proteins such as Bcl-xl and regulate apoptosis by influencing their process, controlling the release of reactive oxygen species (ROS) and cytochrome, regulating molecular factors such as cyclin affecting the cell cycle. On the other hand, curcumin can damage the nuclear and mitochondrial DNA structure of liver cancer cells, thereby disrupting their function [143]. In comparison with free curcumin, the application of liposomal curcumin improves pharmacokinetics and pharmacodynamics while reducing the dosage required to target tumors. Matheus Andrade Chave et al. explored curcumin-containing liposomes by inserting curcumin molecules into the MLV liposome [144]. The synthesis of liposomal curcumin and curcumin structure are described in Fig. 10.
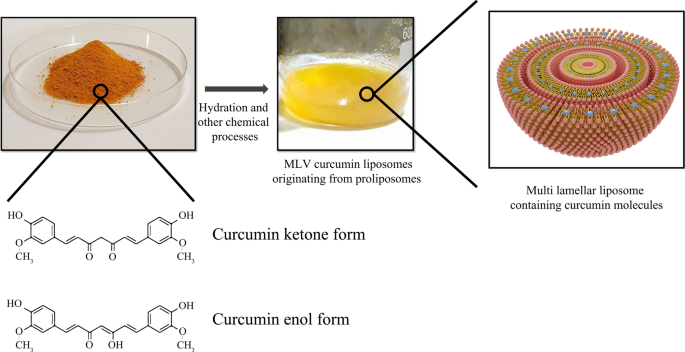
An overview of curcumin powder and liposomal curcumin synthesis. Chemical reactions performed for liposomal curcumin production and curcumin molecule structure in various forms are simply demonstrated
In addition, liposomes prepared for therapeutic research applications can be synthesized by employing various methods. For example, Qiao Wang et al. exploited the ultrasonication and lipid film-hydration method to synthesize daidzein long-circulating liposomes (DLCL) [145]. Xiaoyuan Ding et al. also used the film hydration method for the synthesis of aptamer and Au-NPs (Apt-Au)-modified Morin pH-sensitive liposome. Their outcomes showed high biocompatibility and insignificant toxicity of these liposomal structures and highlighted these liposomes as a viable option for selective targeting of tumors [146].
Other Liposomal NPs in the Investigational Phase
Several liposomal drugs have been synthesized and utilized in various medications at the investigational phase. For instance, CPX-1 was produced by entrapping the antitumor agents, Irinotecan and floxuridine (1:1 molar ratio) in liposomes, and was designed to treat advanced colorectal cancer. This therapeutic nanoparticle is in phase II research status [128]. Lipovaxin-MM is another momentous liposomal nanoparticle in phase I research prepared by placing melanoma antigens in liposomes and mainly administrated for immunotherapy of malignant melanoma. This agent is also under investigation [128].
Conclusion
As spherical structures in liquids, liposomes can be applied as a promising option for cancer therapy and drug delivery, as well as imaging, and disease management. By reviewing liposomes pros and cons, scientists will be able to improve them in future research works.
Some opportunities and challenges in liposomes utilization are described in the following. One of the convenient features of liposomes is their morphological similarity to cells (presence of phospholipids), as well as increasing the effectiveness of the drugs. As a negative point, liposomal phospholipids may sometimes undergo hydrolysis or oxidation reactions which may be problematic. Other pros of liposomes include increased stability of the encapsulated drug in it, reduced contact of sensitive tissues with therapeutic molecules, decreased drug toxicity, improved pharmacokinetic and pharmacodynamics properties, the ability to regulate the rate of drug release, and the potential of their structure to accept the desired chemical modification. In contrast to these opportunities, there are some challenges such as leakage or unintended entrapment of drugs, low liposome bioactivity, decreased-solubility, rapid clearance of conventional liposomes from the blood by the reticuloendothelial system (RES), and problems caused by continuous intravenous administration or local injection.
Besides examining the advantages and disadvantages of liposomes, we should take their proper targeting mechanism of action into account. Passive targeting is considered a beneficial mechanism due to the abundant clinical evidence and experience. It also increases the circulation time of liposomal drugs. The problem of this mechanism lies in its non-specific drug delivery and its physiological barriers. In contrast, beneficial features of active targeting include increased specificity in drug delivery, the possibility of overcoming chemotherapy-resistant tumor cells, and reduced off-target effects. However, the difficulty in identifying accurate binding sites on cancer cells and the lack of adequate evidence of its former utilization have led to some ups and downs in its application.
Liposomes are reasonable candidates for elevating the effectiveness of current anticancer agents and preventing the incidence of drug resistance. Future research in this area should be focused on further investigation into the properties of liposomal structures. To probe about drug entrapment in therapeutic nanoparticles, including liposomes, much more detailed examinations will be required.
缩写
- WHO:
-
World Health Organization
- VNPs:
-
Viral nanoparticles
- NP:
-
纳米粒子
- US FDA:
-
United States Food and Drug Administration
- DDSs:
-
Drug delivery systems
- PC:
-
Phosphatidylcholine
- SM:
-
Sphingomyelin
- 附注:
-
Phosphatidylserine
- PE:
-
Phosphatidylethanolamine
- ULVs:
-
Unilamellar vesicles
- OLVs:
-
Oligo lamellar vesicles
- MLVs:
-
Multilamellar vesicles
- SUVs:
-
Small unilamellar vesicles
- RBF:
-
Round-bottom flask
- LNs:
-
Liposomal nanoparticles
- MPS:
-
Mononuclear phagocytosis system
- PEG:
-
Polyethylene glycol
- PEOZ:
-
Poly [2-ethyl 2-oxazoline]
- PETOXylated:
-
Poly [2-ethyl-2-oxazolylated
- PMOZ:
-
Poly [2-methyloxazoline]
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- SCLC:
-
Small cell lung cancer
- GRPR:
-
Gastrin-releasing peptide receptor
- Anti-EpCAM:
-
Anti-epithelial cell adhesion molecule
- IUPAC:
-
International Union of Pure and Applied Chemistry
- I.V.:
-
Intravenous
- S.C.:
-
Subcutaneous
- I.D.:
-
Intradermal
- I.P.:
-
Intraperitoneal
- I.M.:
-
Intramuscular
- EPR:
-
增强渗透性和滞留性
- fR:
-
Folate receptor
- TfR:
-
Transferrin receptor
- EGFR:
-
Epidermal growth factor receptor
- VEGF:
-
Vascular endothelial growth factor
- VCAM:
-
Vascular cell adhesion protein
- APC:
-
Antigen-presenting cells
- ABCB1:
-
ATP-binding cassette transporter superfamily B member 1
- HSPC:
-
Hydro soy phosphatidylcholine
- DSPG:
-
1,2-Distearoyl-sn-glycero-3-PG
- DOPC:
-
Dioleoylphosphatidylcholine
- DPPG:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol
- DSPC:
-
1,2-Distearoyl-sn-glycero-3-phosphocholine
- AML:
-
Acute myeloid leukemia
- ALL:
-
Acute lymphocytic leukemia
- DSPE:
-
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine
- DOPE:
-
Dioleoylphosphatidylethanolamine;
- EPG:
-
Esterified propoxylated glycerols;
- DMPC:
-
1,2-Dimyristoyl-sn-glycero-3-phosphocholine
- DOPS:
-
1,2-Dioleoyl-sn-glycero-3-phospho-L-serine
- POPC:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DMPG:
-
1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol
- MPEG:
-
Methoxypolyethylene glycols.
- 中枢神经系统:
-
中枢神经系统
- BBB:
-
血脑屏障
- siRNA:
-
Small-interfering RNA
- EphA2:
-
Ephrin type-A receptor 2
- FAK:
-
Focal adhesion kinase
- IL-8:
-
Interleukin-8
- TMRRS/ERG:
-
TMRRS/ERG TROJAN Mobile Remote Receiving System/erythroblast transformation-specific
- EF2K:
-
Elongation factor 2 kinase
- DOTAP:
-
Dioleoyl-3-trimethylammonium propane
- ROS:
-
活性氧
- DLDC:
-
Daidzein long-circulating liposomes
- RES:
-
Reticuloendothelial system
纳米材料


