纳米粒子:升级癌症诊断和治疗的新方法
摘要
传统的癌症疗法因各种副作用和对靶向肿瘤的损伤不足而饱受诟病。纳米粒子的突破为升级传统治疗和诊断提供了一种新方法。实际上,纳米粒子不仅可以解决传统癌症诊治的不足,还可以为肿瘤诊治创造全新的视角和尖端设备。然而,关于纳米粒子的研究大多停留在体内和体外阶段,关于纳米粒子的临床研究报道较少。在这篇综述中,我们首先总结了纳米粒子在癌症诊断和治疗中的当前应用。之后,我们提出了阻碍NPs临床应用的挑战,并结合近两年更新的文献提供了可行的解决方案。最后,我们将对NPs在肿瘤诊断和治疗中的未来发展发表我们的看法。
介绍
全球肿瘤的发病率和死亡率仍然很高。每年有近1400万新癌症患者和800万人死于癌症相关疾病[1]。近年来,传统的肿瘤治疗,如化疗、靶向治疗、放疗、手术等,因进展缓慢、不良反应多、治疗效果不理想等问题不断受到诟病。由于传统肿瘤疗法的不足,越来越多的研究开始寻求靶向能力强、肿瘤干细胞杀伤能力强、不良反应轻微的新型肿瘤治疗方法。新的肿瘤治疗方法包括但不限于免疫疗法、靶向疗法、物理消融、基因疗法、光动力疗法(PDT)和光热疗法(PTT),与传统的肿瘤疗法相比,其疗效显着。这里的处理方法都有一个共同的特点,需要载体配合。虽然病毒可以作为载体,但病毒载体已被证实会引起插入突变和免疫原性[2]。因此,寻找更安全、更有效的载体成为当务之急。
由于纳米粒子的体积小、生物安全性、载药量和物理特性可以辅助物理治疗,因此纳米粒子越来越多地被用作新的肿瘤治疗方法中的载体。这些纳米粒子介导的疗法具有多功能、不良反应少、疗效好的优点[3]。此外,许多由纳米粒子介导的医学成像技术也具有更好的清晰度和准确性,有助于肿瘤的准确诊断[4]。随着纳米技术和医学技术的发展,金、银、铁、脂质体等金属和生物材料在医用纳米粒子(NPs)的生产中得到了广泛的应用[5]。目前,许多研究人员根据这些材料的物理、化学和/或生物学特性,将药物、显像剂甚至基因嵌入纳米粒子中,从而扩展了现有的肿瘤诊断和治疗领域,例如药物靶向递送、增强成像、冷冻手术、PTT 和 PDT [6]。
此外,还有一个现象是大多数纳米粒子只停留在体内和体外阶段。然而,目前缺乏文献总结阻碍 NPs 临床应用的原因。因此,本文旨在总结纳米粒子在肿瘤诊疗领域的应用现状,寻找阻碍纳米粒子进入临床应用的因素,并提出可行的解决方案。
医用功能纳米颗粒的制备和表征
医学上常用的纳米粒子按其组成材料和功能可分为金属纳米粒子、非金属纳米粒子和复合纳米粒子三类,其理化性质受尺寸、形状等参数影响。因此,针对纳米粒子在不同应用方向的功能需求,选择合适的制备工艺非常重要。纳米粒子的所有制备方法可分为两种方法:自底向上法和自顶向下法。自底向上的方法本质上是通过基本单元(原子、分子甚至更小的粒子可以作为组装所需纳米结构的基础)相互堆叠形成纳米粒子,而自顶向下的方法本质上是整个固体材料开始分解成纳米颗粒[7]。表1列出了一些制备医用纳米粒子的例子。
在医学上常用的三种纳米粒子中,金属纳米粒子的应用最为广泛。金属纳米粒子材料包括金属和金属氧化物。金属纳米粒子最常用的制备工艺是日本科学家杉本等人提出的溶胶-凝胶(Sol-Gel)工艺。 20世纪90年代,常用于液相制备单分散金属氧化物颗粒。溶胶-凝胶法是一种自下而上的制备过程。这种制备金属纳米粒子的方法的主要原理是通过化学和物理手段形成金属离子均匀分散的溶胶,然后通过氧化还原反应形成凝胶。凝胶中产生的金属纳米颗粒可以可控地成核、生长和沉积。只要控制实验所用金属胶体的单分散性,控制金属离子与氧化剂/还原剂的浓度关系,就可以控制合成的金属纳米粒子的尺寸。图1为溶胶-凝胶法示意图。
<图片>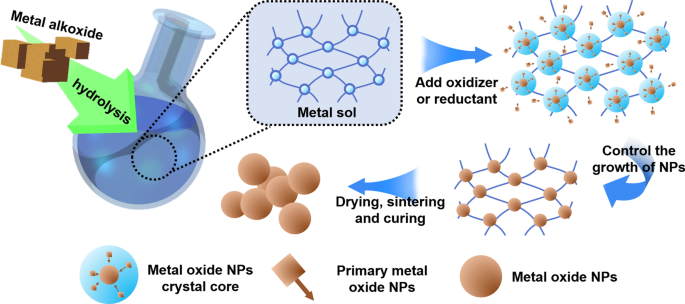
溶胶-凝胶法示意图
常用的自下而上制备金属纳米粒子的方法包括共沉淀法、水热法和光化学法。共沉积法是在液体环境中同时成核、生长和聚集的过程。当溶液过饱和时,会得到大量的小颗粒不溶产物[15]。水热法是在液体环境中进行的过程,通过控制施加到溶液中材料的蒸气压来控制所得纳米颗粒的形态。此外,还有一些自顶向下的制备金属纳米粒子的方法,如电线爆炸法和球磨法。电线爆炸的原理是在电爆炸过程中,金属原子在电解液中蒸发并迅速冷却,形成氧化物纳米颗粒。通过控制电解质组成和电流强度,可以控制更细更均匀的纳米粒子。球磨是利用铣削行星齿轮等加工工具,通过选择合适的磨削时间和相关设备工艺参数,快速、大规模生产尺寸可控的纳米颗粒的方法。除金属纳米粒子外,该制备方法还可应用于其他类型的纳米粒子。
第二种常见类型是非金属纳米粒子。医学中常用的非金属纳米粒子包括聚合物纳米粒子、生物分子衍生的纳米粒子、碳基纳米粒子和二氧化硅纳米粒子 [16,17,18]。其中,二氧化硅纳米粒子最具代表性。硅胶表面有丰富的羟基,有利于探针或荧光基团在表面的结合,因此具有更灵活的功能。二氧化硅纳米粒子的常用合成方法是溶胶-凝胶法和Stöber 法[19, 20]。经典的Stöber法是通过硅酸盐在碱性条件下水解缩合制备二氧化硅纳米粒子,简单高效。
随着纳米技术的发展,复合纳米粒子因其优越的功能相容性而被开发出来。金属纳米粒子具有许多非金属纳米粒子所不具备的特性,如等离子体共振效应(SPR)、磁场中的可控性等,但金属粒子在体内难以有效降解,过度使用具有一定的毒性到细胞 [21]。因此,通过不同的制备方法将不同材料的纳米粒子组合成复合纳米粒子,可以实现功能扩展。魏等人。制备金纳米棒(Au NRs),然后在Au NRs 上进行N-异丙基丙烯酰胺(NIPAAM)的表面引发原子转移自由基聚合(SI-ATRP)以合成近红外响应纳米杂化物[22]。这种结合金属和高分子材料的复合纳米颗粒同时具有光热和近红外光对应的药物释放能力。包络的水凝胶壳使这种纳米颗粒比单个 Au 纳米颗粒具有更好的生物相容性。 Prakash通过改进的Stöber方法合成了以Au为核、SiO2为壳的复合纳米颗粒。核壳纳米粒子的惰性壳层有利于降低金属粒子的毒性,提高原始单金属纳米粒子的材料稳定性和载药能力[23]。
除了上述传统的纳米粒子制备方法外,随着纳米技术科学的发展,对生态和环境保护提出了新的要求,因此出现了新的环境友好型纳米粒子合成方法[24]。 Hajar 等人第一次。使用甜叶菊作为生物还原剂,成功合成了粒径为 1 至 40 nm 的 ZnS 纳米颗粒。以这种方式合成的硫化锌纳米粒子具有良好的生物相容性[25]。根据绿色化学的原理,Miri 等人。使用 P. farcta(豆科植物)提取物快速合成粒径约为 30 nm 的 CeO2 NP。这种纳米粒子具有良好的生物相容性[26]。
用于医学成像的纳米粒子
医学影像在肿瘤的诊断和治疗中发挥着重要作用。许多纳米粒子,如氧化铁纳米粒子,具有可增强成像的光学、磁性、声学和结构特性(图 2)。一些研究表明,将NPs引入靶组织可以提高图像对比度,为肿瘤手术和诊断提供更好的图像指导[27]。例如,在冷冻手术中,NPs可以提高肿瘤和冰球边缘的成像质量,有助于准确覆盖冰球,提高治疗效果[28]。此外,大多数用于成像的纳米粒子都是由金属制成的。根据不同成像原理的不同,纳米粒子也将由不同的金属材料制成。表 2 列出了一些最近关于由不同材料制成的用于医学成像的纳米颗粒的例子。
<图片>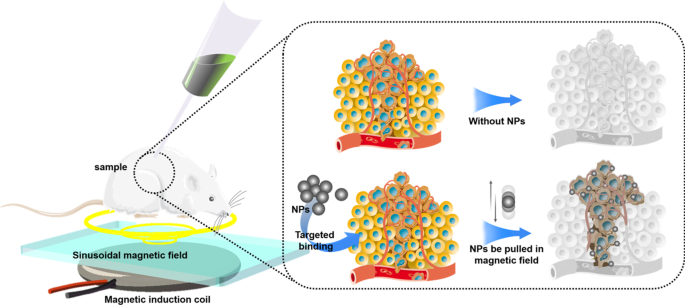
NPs成像改进示意图
光学相干断层扫描 (OCT) 是一种非侵入性、微米级分辨率和生物医学成像技术。 OCT 可用于实时诊断和手术指导。然而,OCT 无法检测非弹性散射光,因为这种光在入射场中不相干 [35]。最近,许多研究证明NPs的运动状态可以改变OCT的幅度,这可能会解决这个问题。干扰 NP 通过磁场的运动会导致光散射的局部变化。有研究指出,将磁性纳米粒子置于磁场中控制其运动,可以改变该区域的光散射,从而可以检测到原本不相干的非弹性散射光。这种新的成像方法是磁动光学相干断层扫描(MMOCT)[36]。
MRI是最有效的无创肿瘤检测技术之一。然而,生物学背景和癌组织之间缺乏MRI信号比较往往影响临床肿瘤诊断[37]。 MRI是一种测量水分子中氢分子磁化强度的扫描成像方法。每个解剖结构呈现不同的图像,因为每个组织的质子会引起不同的磁化变化。可以通过应用更多的造影剂来提高图像的可见性 [38, 39]。广泛用于肿瘤早期检测的肿瘤相关 EPR 效应对磁性 NPs 产生了巨大的对比度增强能力 [40]。氧化铁磁性纳米颗粒 (IONPs) 是目前最常见的 MRI 纳米探针造影剂,具有一定的细胞靶向性 [41]。例如,研究发现 IONPs 在使用 MRI 诊断肝癌时可以进入健康肝脏 Kupffer 细胞,但会被排除在癌细胞之外,导致低信号健康组织和高信号肿瘤组织 [42]。基于最近的研究,适当的粒子表面修饰和适当的肿瘤特异性生物寡聚体嵌入 NPs 可以更好地固定肿瘤中的 NPs 以获得更清晰的成像结果,甚至可以用于早期的微肿瘤成像。例如,研究发现靶向人转铁蛋白的AuNPs可以显着增强脑肿瘤的成像效果[43]。高等人。在顺磁性纳米颗粒探针的基础上,搭载抗表皮生长因子受体单克隆抗体(mAb)实现小肿瘤的成像[44]。
用于靶向给药的纳米颗粒
化疗药物虽然是目前最常用的肿瘤治疗方法,但仍存在恶性肿瘤区域靶点富集差、健康组织过度蓄积等问题[45]。这可能会导致骨髓、毛囊、胃肠道细胞和淋巴细胞等细胞分裂强烈受到抑制,导致骨髓抑制、粘膜炎、脱发甚至死亡等不良反应[46]。靶向给药是指主动分化正常细胞和癌细胞进行给药,比常规治疗具有更好的疗效和更少的不良反应[45]。许多研究证实,NPs可以通过主动或被动靶向将化疗药物靶向肿瘤细胞[47]。此外,许多实验发现NPs在免疫药物的靶向递送中也发挥着重要作用[48]。
如图 3 所示,被动靶向通常依赖于肿瘤组织的一些病理生理特征,包括异常血管、温度、pH 和肿瘤细胞的表面电荷 [49]。例如,由于增强的渗透性和保留效应(EPR ) 肿瘤组织中的血管,直径约 400 nm 的 NPs 可以被动转移到肿瘤组织 [50]。然而,被动靶向方法在 NPs 的物理化学性质方面存在许多限制,例如直径、表面电荷、分子量、疏水性或亲水性。此外,被动靶向技术在药物扩散效率方面表现不佳,并且在肿瘤细胞中显示出不足的 EPR 效果 [51]。由于被动靶向的不足,近年来关于药物递送NPs的研究大多转向主动靶向(配体靶向)。表 3 突出显示了一些最近用于药物递送的 NPs 示例。
<图片>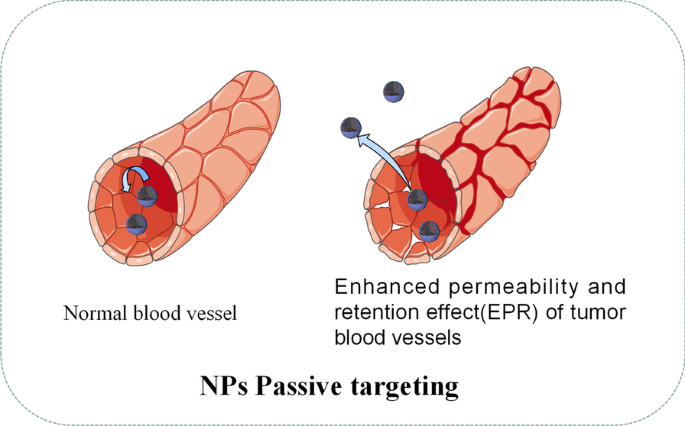
NPs被动瞄准示意图
主动靶向(配体靶向)NPs 通常携带一些肿瘤特异性生物标志物的配体 [61]。如图 4 所示,当配体与肿瘤表面的受体接触时,NPs 可以通过受体介导的内吞作用被肿瘤细胞内化,并由于细胞内环境中的酸性 pH 和特定酶而释放药物。 62]。对于靶向配体,目前研究中普遍使用叶酸、转铁蛋白、表皮生长因子受体(EGFR)和糖蛋白[62]。例如,桑多瓦尔等人。通过配备吉西他滨的 EGFR 靶向硬脂基 NP,观察到显着的药物富集和显着的治疗乳腺癌小鼠的疗效 [63]。潘迪等人。发现携带盐酸小檗碱(BHC)的叶酸靶向金纳米粒可以有效地将药物递送至表达叶酸受体的人宫颈癌细胞[64]。
<图片>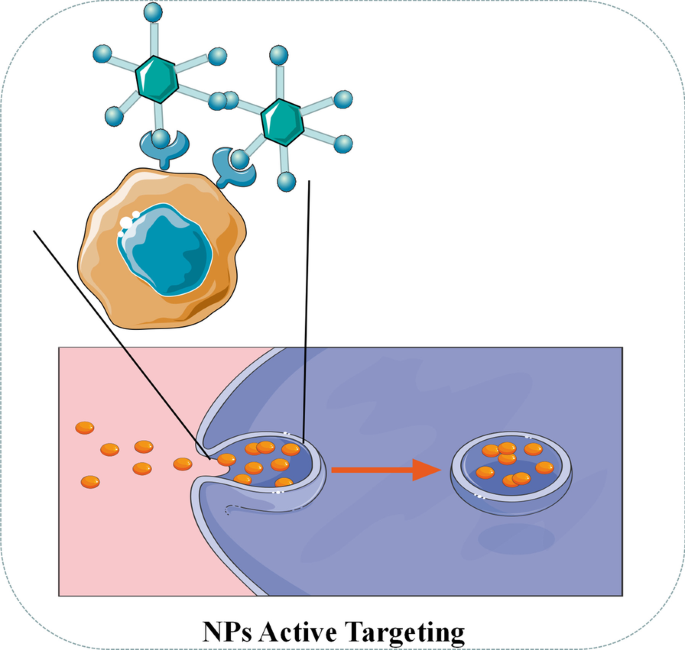
NPs 主动靶向示意图
近年来,与化疗药物相比,短干扰RNA(siRNA)介导的基因沉默疗法被视为肿瘤治疗的新前景[64]。尽管病毒可用作 siRNA 的传递载体,但已证实病毒载体会引起插入诱变和免疫原性 [65]。相比之下,据报道硒纳米颗粒作为 siRNA 载体具有巨大潜力,因为微量元素硒本身可以减少肿瘤的发生,降低药物毒性,并调节免疫功能 [66]。此外,硒纳米颗粒的表面可以负载各种肿瘤靶向部分(如叶酸、透明质酸和 RGD 肽)以增强肿瘤靶向能力 [67]。夏等人。据报道,RGDfC 肽靶向的硒 NPs (RGDfC-Se@siRNA) 具有出色的靶向 HeLa 宫颈癌的能力 [60]。同时,由于RGDfC可以与多种肿瘤细胞高表达的αvβ3整合素特异性结合,RGDfC-Se@siRNA NPs可以重复用于多种肿瘤的靶向给药[68]。在结构方面,带有正电荷的 RGDfC-SeNPs 可以通过它们的静电相互作用将带负电荷的 siRNA 紧密包裹起来 [69]。通过动物实验,RGDfC-Se@siRNA NPs 显示出通过网格蛋白相关的内吞作用有效进入肿瘤细胞的能力。在肿瘤细胞中,它可以快速释放siRNA并有效沉默相关基因,促进活性氧(ROS)的产生,从而抑制肿瘤细胞增殖,促进肿瘤细胞凋亡[69]。此外,多种SeNPs均表现出优异的生物安全性,对小鼠肝、肾、心、肺、脾等主要器官无明显毒性损伤[60,70,71]。
目前,虽然有很多用于靶向给药的纳米颗粒,但大部分应用仍停留在细胞或动物实验阶段,缺乏有力的临床应用支持。此外,许多纳米颗粒是瘤内给药,限制了纳米颗粒适用于肿瘤的范围,缺乏特殊的纳米颗粒给药工具和其他给药方式。
因此,探索更好的NPs给药方式可能是未来靶向给药NPs研究的一个方向。根据现有的学术期刊,血管介入给药可能是一种可行的方式。在假设中,首先借助成像定位供瘤血管的位置,然后使用导丝将纳米颗粒直接引入供瘤血管中,并通过应用控制纳米颗粒在小范围内的运动。同时产生磁场。因此,NPs 可以固定在适当的位置,而不受血管中血流的影响。否则,靶向给药的 NPs 仅具有一定的局限性。靶向NPs会影响化疗药物的全身分布,降低化疗对游离肿瘤细胞和微转移的影响。如果配上靶向药物,靶向作用有增强的趋势,而根据现有研究,改善并不明显。另外,抗肿瘤药物不太可能自行清除所有的肿瘤干细胞。然而,基于纳米粒物理特性的物理疗法往往对肿瘤干细胞更有效。因此,未来倾向于以药物载体为靶点的多功能纳米颗粒,如冷冻手术、光热疗法(PTT)和光动力疗法(PDT)等,形成多功能纳米颗粒用于肿瘤治疗。
用于冷冻手术的纳米粒子
冷冻手术是一种通过冷冻破坏肿瘤组织的技术,具有侵入性小、成本低、术中出血少、术后并发症少等优点,但仍存在冷冻效率不足、冷冻损伤周围组织等缺点[28]。尽管使用了抗冻蛋白(AFP-1)等保护剂来辅助冷消融,但效果仍不理想[72]。随着纳米技术的发展,提出了纳米冷冻手术的概念。纳米冷冻手术的基本机制是将具有特定物理或化学特性的纳米颗粒引入肿瘤组织中。利用NPs的特性,不仅可以提高冷冻的效率和效果,还可以控制范围调整和冰球形成的方向。因此,纳米冷冻手术能够同时杀死肿瘤组织并防止周围健康组织被冷冻[73]。纳米冷冻手术的优势如图5所示。
<图片>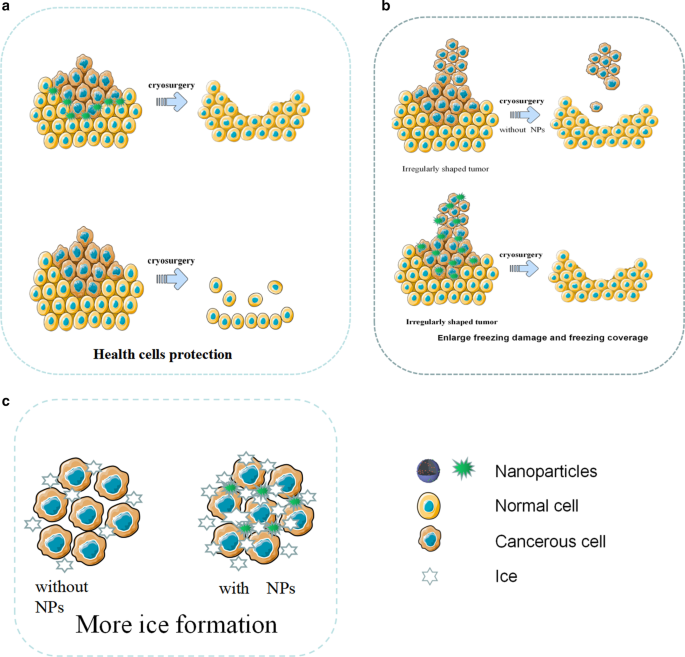
用于冷冻手术的 NP 的示意图。 一 NPs 在冷冻手术期间保护健康细胞。 b NPs 增强冻结损伤并控制冻结覆盖率。 c 在NPs的帮助下,形成了更多的冰
在冷冻手术中,细胞内结冰是肿瘤细胞损伤的关键。同时,研究证明NPs可以有效诱导细胞内冰的形成[28]。纳米粒子作为外部粒子可以诱导异质成核。研究发现,富含 NPs 的组织比传统组织冷冻得更快,并且更容易发生异质成核。在相同的冷冻条件下,含有 NPs 的组织更容易结冰,说明 NPs 可以显着提高细胞内结冰的速度和概率,可以更有效地杀死肿瘤细胞 [74]。此外,含有金属氧化物的纳米颗粒将显着提高肿瘤组织的热导率。例如,Liu 和 Deng 比较了含有和不含 NPs 的猪肉组织的温度响应曲线。他们发现含有NPs的组织冷却迅速,最低温度可达115℃,远低于不含NPs的对照组。
由于肿瘤通常形状不规则,传统冷冻手术产生的冰晶往往无法覆盖所有肿瘤组织。与传统的冷冻手术相比,纳米冷冻手术可以轻松解决这个问题。由于纳米颗粒可以渗透到细胞内液中并具有良好的导热等物理特性,因此可以通过纳米颗粒的分布来控制冰球的生长方向和方向[73]。
在冷冻手术中,冷冻不足可能不会完全破坏肿瘤组织,过度冷冻可能会损伤邻近的健康组织。尤其是当肿瘤与脆弱器官密切接触、位置较深或形状不规则时,对健康组织的损害尤为严重。近年来,由 NPs 制成的相变材料 (PCM) 已在冷冻手术过程中显示出对周围健康组织的出色保护潜力 [75]。例如,Lv 等人。微囊化相变NPs通过脂质体具有大潜热和低导热性,并在冷冻手术前将微囊化相变NPs注射到肿瘤周围的健康组织中,发现避免了对健康组织的低温损伤[76]。
虽然NPs在冷冻手术中得到了广泛的应用,但仍存在一系列的不足。首先,它仍然无法在体外控制纳米颗粒,导致纳米颗粒在肿瘤组织中分布不均,预期功能不理想。其次,虽然有多种磁性纳米粒子,但体外磁场控制纳米粒子的实际效果仍不理想。此外,纳米冷冻手术缺乏临床实验研究,许多纳米颗粒仍处于实验室阶段。
纳米粒子在冷消融中的应用一般可分为协同作用和保护作用两种,两者在纳米粒子的设计要求和体内分布方面有所不同。未来,纳米冷冻手术可能会得到多种纳米颗粒的辅助,即协同纳米颗粒分布在肿瘤内部,而保护纳米颗粒分布在肿瘤周围。此外,许多纳米定位装置,例如目前用于放射性粒子植入前肿瘤定位的穿刺设计的3D打印共面模板(3DPCT),也可用于冷冻手术。在冷冻手术之前,可以通过 3D 打印共面模板 (3DPCT) 和 CT 引导在肿瘤周围穿刺并注射保护性纳米颗粒,以保护周围的健康组织。 NPs 能够协助冷冻手术冰球覆盖肿瘤的不规则边缘。然后通过预设的消融部位穿刺或血管介入,将协同的NPs导入肿瘤组织进行冷消融。这种纳米冷冻技术不仅可以克服不规则肿瘤冷消融的困难,还可以增加冷消融的效果,减少对健康组织的损伤。这种方法可能会成为纳米冷冻手术未来的研究方向。表 4 突出显示了最近用于冷冻手术的 NPs 的一些例子。
用于 PTT 和 PDT 的纳米粒子
目前,基于纳米粒子 (NPs) 的光热疗法 (PTT) 和光动力疗法 (PDT) 已显示出在肿瘤治疗过程中疗效强、侵袭小、副作用轻的优点(图 6)[80]。除了直接杀死肿瘤细胞外,PDT 和 PTT 治疗产生的死亡肿瘤细胞碎片还可以作为潜在抗原触发持续的免疫反应,称为光热和光动力免疫治疗 [81]。基于PTT治疗理念设计的纳米粒子是一种新型的光热转换纳米材料,可以将光能转化为热能杀死癌细胞。与传统的光热转换材料相比,纳米粒子具有诸多优势。首先,NPs可以通过颗粒表面修饰达到肿瘤靶向聚集的效果,有助于提高靶肿瘤的富集能力[82, 83]。其次,纳米粒子比传统的光热材料具有更好的成像能力,可以通过CT、MRI和光声成像准确定位[84, 85]。 Pan等人合成的靶向纳米粒子。可以在 0.2 W/cm 2 下进行 PTT NIR 通过破坏肿瘤细胞核 DNA 和抑制 DNA 修复过程来诱导肿瘤细胞凋亡 [86]。表 5 列出了最近在 PDT 和 PTT 中使用的 NPs 的一些例子。
<图片>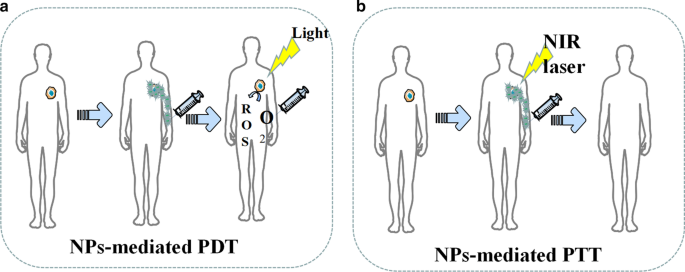
NPs 介导的 PDT 和 PTT 的示意图。 一 NPs促进活性氧的产生。 b NPs增强PTT过程中的肿瘤损伤
此外,一些研究发现纳米颗粒介导的PTT可以逆转肿瘤多药耐药(MDR)。药物转运蛋白、多药耐药相关蛋白 1 (MRP1) 和 p-糖蛋白 (p-gp) 的过度表达通常被认为会在各种肿瘤中引起 MDR [95]。例如,Li 等人设计的多功能光触发纳米粒子。可以抑制 PTT 中 MRP1 的表达,从而逆转 A549R 细胞的耐药性 [96]。王等人。据报道,金纳米粒子和碳基纳米粒子都可以通过促进 PTT 中热休克因子三聚体的表达来克服 DOX 抗性,从而抑制 p-gp 的产生 [97, 98]。此外,纳米粒子介导的PTT还可以通过破坏肿瘤细胞膜的完整性来提高化疗的有效性[99]。
PDT is a treatment that uses the selective retention of photosensitizing substances (PSs) in tumor tissue under the activation of specific wavelength excitation light and the presence of molecular oxygen to produce singlet oxygen and other reactive oxygen species, which leads to tumor cell apoptosis and necrosis [100]. However, traditional PS has poor tumor targeting, poor solubility, and instability, which is vulnerable to the internal environment [100]. Nanoparticle carriers modified by targeted molecules can not only improve the stability and biocompatibility of PS but also deliver PS to target cells, which improves the efficacy and reduces adverse effects [100]. Additionally, some common nanomaterials, like gold nanorods, have excellent PTT effects themselves. For example, Vankayala et al. found that the exposure of gold nanorods to near infra-red light (915 nm) were able to efficiently induce the generation of singlet oxygen [100].
In recent years, the role of up-conversion (UC) nanoparticles in PDT has attracted much attention. The NPs can convert long-wavelength light excitation into multiple short wavelengths, which enables the UC to replace the traditional ps-dependent short-wavelength excitation light with the near-infrared light with strong tissue penetration ability [101].例如,李等人。 developed dual-band luminescent lanthanide nanoparticles as a PS carrier. This UC nanoparticles rely on the excitation light wavelength of 808 nm to achieve image-guided PDT without affecting imaging signals [102].
Since most photosensitive materials utilized in the phototherapy are metals, the biocompatibility of NPs designed for inorganic nanomaterials like metal ions still needs to be improved.
NPs-mediated phototherapy is now credited for not only the effectiveness against tumor but also the potential for spare internal space of nanoparticles since the therapy only utilizes the physical properties of NPs skeleton. Therefore, NPs are often multifunctioned by PDT and PTT. In the future, such NPs may be designed as dedicated NPs for tumor stem cells that are not sensitive to chemotherapy. Tumor stem cells are dormant for a long time and have a variety of drug-resistant molecules, so it is difficult to kill them by conventional treatments like chemotherapy, whereas the light therapy is more effective by killing the tumor stem cells physically. In the future, nanophysical therapy may be used with many other techniques, such as the multifunctional NPs for photothermal therapy after cryosurgery. Multifunctional NPs mediated therapy can give full play to its characteristics of low side effects, strong local lethality, and tumor stem cell killing. In addition, because nano-physiotherapy has a local killing effect and can effectively kill tumor stem cells, it may become a treatment method for small metastases.
Nanoparticles for Radiotherapy
Radiotherapy (RT) is a tumor treatment technique that kills local cells by ionizing radiation generated by rays and is currently an effective treatment for many primary and metastatic solid tumors [103]. Experiments prove that radiotherapy can effectively kill tumor stem cells [104].However, how to further improve the efficacy of radiotherapy is still a serious challenge. In recent years, nanoparticles in the field of radiotherapy have demonstrated strong radiosensitization capabilities, tumor-targeted delivery capabilities of radiosensitizing drugs, and imaging guidance enhancement capabilities [105]. At present, the most popular nanoparticles are made by high Z (atomic number) metal materials, which are featured by chemical inertness and strong radiation absorption capacity. They produce various reactions such as photoelectric effect and Compton effect after absorbing radiation, thereby releasing a variety of particles such as optoelectronics, Compton electrons, and Auger electrons. These electrons react with organic molecules or water in tumor cells to generate a large number of free radicals, leading to synergistic chemotherapy [106]. Common chemotherapy-sensitized NPs are currentlycategorized as precious metals, iron oxides, and semiconductors in terms of materials.
Precious metals NPs are made of high atomic number metal materials such as gold, silver, gadolinium, hafnium, platinum, bismuth, etc. [107]. Among them, gold nanoparticles have become the most popular NPs due to their good biocompatibility, chemical stability, and relatively strong photoelectric absorption coefficient [108]. In 2000, Herold et al. discovered the chemosensitizing ability of gold nanoparticles in kilovoltage X-rays. Nowadays, the specific mechanism of chemosensitization of gold nanoparticles is not yet clear, and the mainstream view believes that it depends on the photoelectric absorption capacity of high atomic number [109]. In addition to this, there are studies suggesting that the presence of gold nanoparticles improve the chemical sensitization of DNA to radiation, which increases the DNA damage induced by ionizing radiation (IR). At the same time, gold NPs can catalyze the mechanism of radiotherapy sensitization such as free radical production [105]. For instance, Liu found that AuNPs could significantly increase the production of hydroxyl radicals as well as the killing effect of x-rays and fast carbon ions on cells [110]. The hypothesis of the chemotherapy sensitization mechanism of other precious metals is similar to that of gold nanoparticles. Particularly, platinum NPs have an anti-tumor effect due to the inherent nature. Consequently, platinum NPs are expected to play the role of chemotherapy and radiotherapy simultaneously. However, the number of relevant research reports is insufficient, and the sensitizing effect of platinum NPs is also questionable. For example, Charest et al. reported that liposomal formulation of cisplatin was able to increase the uptake of platinum by tumor cells, and could enhance the killing of F98 glioma cells by γ-rays at the same time [111]. On the contrary, Jawaid et al. reported that platinum NPs would reduce the generation of reactive oxygen species (ROS) and the efficacy of radiotherapy during chemotherapy [112].
Iron oxide nanoparticles (IONs), especially the superparamagnetic magnet Fe3O4, have shown great potential in image-guided tumor radiotherapy because they are capable of enhancing the dose of radiotherapy and MRI imaging, whereas its sensitization mechanism is not clear yet. Its sensitization mechanism is not yet clear. Some studies believe that iron oxide NPs mainly catalyze the generation of ROS through Fenton's reaction and Haber–Weiss reaction. Then the highly reactive ROS will kill tumors [112,113,115]. Other studies propose that the mechanism depends on the radiation sensitization and synergistic effects of magnetic nanoparticles. As Khoei reported, iron oxide NPs can improve the radiosensitization of prostate cancer cells in vitro [116].黄等人。 pointed out that cross-linked dextran-coated IONs (CLIONs) could be internalized by HeLa cells and EMT-6 mouse breast cancer cells, which enhances radiation therapy [117]. Although the synergistic effect of iron oxide NPs is obvious, its biological safety still needs to be improved. Many studies have proved that the biocompatibility and chemical stability of iron oxide NPs are questionable, and it has certain toxicity [118].
Semiconductor NPs like silica NPs have also been found to have a synergistic effect on radiotherapy.例如,张等人。 used flow cytometry analysis and MTT experiments to find that mesoporous silica NPs can effectively enhance the radiotherapy of glioblastoma [119].他等人。 reported the mechanism of radioactive enhancement of silica NPs. He found that under X-ray irradiation, silica nanoparticles could produce fine hydroxyl radicals, which can effectively kill tumor cells [120].
At present, although many experiments have confirmed that NPs were able to sensitize radiotherapy, the specific mechanism of sensitization is still unclear, which hinders the development of new sensitized NPs. There are some doctrines like sensitizing chemotherapy that promotes free radical production. Nevertheless, there is a lack of a quantitative relationship among the amount of free radical production, radiation intensity, and physical data of nanoparticles. In addition, most sensitized NPs are made of high atomic number metals. These metals have many disadvantages in human body such as difficulty in self-metabolism and biodegrading. Meanwhile, long-term accumulation of the metals will produce toxicity, which limits the safe use of radiosensitized NPs. Moreover, compared with the radiotherapy sensitization NPs, fewer studies focused on NPs which can prevent the adverse reactions of radiotherapy and protect healthy tissues. The research on radiotherapy protective NPs is short in quantities.
In the future, searching for NPs material that can be metabolized by the kidney, biometabolized, biocompatible, stable in physicochemical properties, and inherently less toxic, or looking for surface modification that can help the body metabolize NPs may become a research direction for sensitized NPs. Moreover, although there have been many NPs studies on multi-function, namely simultaneous sensitization of radiotherapy and chemotherapy, there are still many potentials in this field, which are worthy of focus in the future. The development of protective NPs that can protect normal tissues around radiotherapy and alleviate poor defense against radiotherapy may also become a research direction.
结论
The poor curative effect, inefficient targeting ability, various side effects, and potential biological risk are some of the unfavorable attributes of conventional cancer therapy and diagnosis. In recent years, advanced nanotechnology and molecular cell biology have promoted the applications of NPs in cancer field. Not only metal NPs, but also many lipid, nucleic acid and silicon NPs showed evident outperformance in cancer diagnosis and treatment.. Moreover, new generation of NPs is no longer limited to solo but multiple functions. For example, gold-coated poly(lactic-co-glycolic acid) (PLGA) NPs equipped with PD-1 blockers which were designed by Luo et al. can not only target drug delivery but also mediate PTT therapy [121]. (Pd @ Au) / Fe3O4 Spirulina NPs with doxorubicin created by Wang et al. demonstrated the functions of photothermal therapy, delivery of chemotherapy drugs, and magnetic field control in cell experiments [122]. Multifunctional nanoparticles will become the trend of future research.
At present, we find that most of the nanoparticles only stay in vivo and in vitro stage. According to this review, we think the following reasons hinder the clinical application of NPs.
- (i)
Lack of injection routes and methods
Most NPs are injected into body via puncture or intravenous injection. Therefore, the blood flow will take away NPs, making NPs difficult to stay in the target area for a long time, which leads to just few NPs that can be uptaked by tumor cells. Low-concentration drugs cannot produce the expected therapeutic effect, and low-concentration NPs also affect the physical killing effects of PDT, PTT, cryosurgery, and radiotherapy. In our opinion, magnetic NPs platform may be a solution. There have been many in vitro and in vivo experiments that have proved the feasibility of using the three-dimensional magnetic field to control the movement of NPs against blood flow [122,123,125]. However, how to solve the interference of the human body to the magnetic field, how to solve the impact of blood cells colliding with NPs, and how to control a large number of NPs in a group are still in discovery.
- (ii)
Difficulty in localization of NPs in vivo
Compared with the human body, the size of NPs is too tiny. Even if NPs are loaded with fluorescent proteins, it is still difficult for conventional imaging equipment (CT, X-ray, MRI) to locate the NPs in the human body in real time. To deal with this challenge, photoacoustic computed tomography (PACT) may be a solution. Photoacoustic computed tomography (PACT) has attained high spatiotemporal resolution (125-μm in-plane resolution and 50-μs frame −1 data acquisition), deep penetration (48-mm tissue penetration in vivo), and anatomical and molecular contrasts [126]. Because of excellent performance, PACT has great potential in NPs localization imaging in vivo. The PACT-guided microrobotic system designed by Wu et al. has achieved controlled propulsion and prolonged cargo retention in vivo of NPs with a diameter of 50 μm [127]. Although the current resolution and deep penetration of PACT are still insufficient, it is superior to conventional imaging equipment (CT, X-ray, MRI) in terms of NPs imaging positioning.
- (iii)
Difficulty of degrading in the human body
Although NPs are made of high biosafety materials, there is still a risk of damages to liver, kidney, and other organs if they stay in the body for a long time and cannot be degraded or excreted The use of materials that will be disintegrated after near-infrared light irradiation to fabricate NPs may be a solution to this problem. Recently, more and more NPs have been produced by these materials. Such NPs mediate PTT while loading drugs, meanwhile, the substances produced by the disintegration of NPs can be rapidly metabolized by the human body. In addition, the use of more biocompatible and degradable materials for nanoparticle preparation is also a solution. For example, the surface of chitosan is positively charged and can be broken down by the colonic flora, which facilitates interaction with specific tissues and can be metabolized by the body. The biocompatibility and degradability of chitosan has been proven to be non-toxic at appropriate drug concentrations [128].
- (iv)
Difficulty in avoiding mononuclear phagocytic system (MPS)
In biofluids, NPs will adsorb proteins to form a corona layer referred to as “protein corona” in a broader sense giving biological identity to NPs and alters their biological characters, which will attract MPS especially macrophages to uptake NPs [129]. In order to avoid being uptaken by MPS, various polymer coatings such as forpolyether, polybetaine (PB) and polyolhave were investigated to cover NPs. For example, polyglycerol-grafting NPs are able to evade macrophage uptake by reducing protein adsorption [130]. In addition, there are two types of tumor-associated macrophages (TAM), M1 and M2. M1 macrophages inhibit tumor growth while M2 macrophages promote tumor growth. Therefore, no longer avoiding macrophages, but designing NPs targeted by macrophages, by regulating the function of macrophages, and even using macrophages as new drug carriers to exert anti-tumor effects may become a novel solution. At present, common design strategies for such NPs include inhibiting macrophage recruitment, depleting TAM, reprogramming TAMs, and blocking CD47-SIRPα pathway [131]. Among them, following the design concept of reprogramming or blocking CD47-SIRPα pathway, NPs that repolarize M2 macrophages to M1 type have made a breakthrough in vivo experiments [132].
Considering the above difficulties and referencing to advanced researches, we come up with a new possible design of NPs. The NPs skeleton is made of pyrolytic material (spirulina, exosomes, et al.). Then, photothermal materials (Au, Pd, etc.) are deposited on the NPs skeleton through electroless plating. After that the superparamagnetic iron oxide will be loaded on the surface of NPs through the sol–gel method. Then, suitable polymers (polybetaine, polyglycerol, etc.) will coat the NPs. Finally, drug (like doxorubicin) will be loaded on the NPs. Afterwards, under the guidance of PACT, NPs will be injected into the upstream of tumor supplying blood vessel, and the tumor will be irradiated with NIR. At the same time, three-dimensional magnetic field control is given to maximize the accumulation of NPs at the tumor site. Through this design, a large number of NPs will accumulate at the tumor site to ensure the drug concentration and PTT effect. At the same time, most NPs will be decomposed at the tumor site, and only a small number of NPs will circulate in the body.
Nowadays, anti-tumor therapy with NPs as the main body is still in the exploratory stage, and related technologies and equipments need to be invented, so it is unlikely to be clinically used in the short term. However, NPs can change part of the function or structure of many actual technologies. The upgrade of actual technologies is expected to be applied in clinic quickly, which contributes to upgrading the diagnosis and treatment of tumors in consequence. For example, NPs can help to develop electrochemical devices based on the interaction between ions and conductive polymers, such as organic electrochemical transistors (OFETs), electrolyte gated field-effect transistors (FETs), fin field-effect transistor (FinFETs), tunneling field-effect transistors (TFETs), electrochemical lab-on-chips (LOCs) [133]. These electrochemical devices are widely used in various tumor testing and diagnostic equipment. The use of NPs can help improve the accuracy of the equipment and reduce the detecting time. Many studies indicate that medical equipment using electronic components upgraded by NPs have been applied clinically [133,134,136].
Based on the evidence cited above, future research of NPs may not only focus on NPs themselves but also consider a feasible administration and efficacy assessing platform. In addition, the platform needs to be able to monitor immunotoxicity, the long-term toxicity, and neurotoxicity of NPs. As nanotechnology develops, if these problems were solved, NPs would be an ideal approach to upgrade cancer therapy and diagnosis.
数据和材料的可用性
All data generated or analysed during this study are included in this published article.
缩写
- NP:
-
纳米粒子
- PDT:
-
Photodynamics therapy
- PTT:
-
光热疗法
- SPR:
-
Plasmon resonance effect
- Au NRs:
-
Gold nanorods
- SI-ATRP:
-
Surface-initiated atom transfer radical polymerization
- NIPAAM:
-
N-isopropylacrylamide
- US:
-
超声
- MSNs:
-
Mesoporous silica nanoparticles
- USMO:
-
Ultrasmall manganese oxide
- GEM:
-
Gemcitabine
- OINPs:
-
Oxygen/indocyanine green-loaded lipid nanoparticles
- PA:
-
Photoacoustic
- MPI:
-
Magnetic particle imaging
- MRI:
-
磁共振成像
- SPIO:
-
Superparamagnetic iron oxide
- USPIO:
-
Ultra-small SPIO
- OCT:
-
Optical coherence tomography
- MMOCT:
-
Magnetomotive optical coherence tomography
- mAb:
-
Monoclonal antibody
- DOX:
-
阿霉素
- 5-FU:
-
5-Fluorouracil
- FA:
-
叶酸
- PTX:
-
Paclitaxel
- ROS:
-
活性氧
- EPR:
-
增强渗透性和滞留作用
- EGFR:
-
Epidermal growth factor receptor
- BHC:
-
Berberine hydrochloride
- AFP-1:
-
Antifreeze protein
- PCMs:
-
Phase change materials
- 3DPCT:
-
3D printed coplanar template
- RCDs:
-
Amino-rich red emissive carbon dots
- COF:
-
Covalent organic framework
- ICG:
-
Indocyanine green
- HSA:
-
Serum albumin
- MDR:
-
多重耐药性
- MRP1:
-
Multidrug resistance-associated protein 1
- p-gp:
-
P-糖蛋白
- PSs:
-
Photosensitizing substances
- UC:
-
Up-conversion
- RT:
-
Radiotherapy
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PACT:
-
Photoacoustic computed tomography
- MPS:
-
Mononuclear phagocytic system
- PB:
-
Polybetaine
- TAM:
-
Tumor-associated macrophages
- OFETs:
-
Organic electrochemical transistors
- FETs:
-
Electrolyte gated field-effect transistors
- FinFETs:
-
Fin field-effect transistor
- TFETs:
-
Tunnelling field-effect transistors
- LOCs:
-
Electrochemical lab-on-chips
纳米材料


