使用 Cu 和 Cu/Cu2O 纳米颗粒和 CuSO4 的基于聚(己二酸丁二醇酯-共聚对苯二甲酸酯)的纳米复合材料和复合材料的抗菌作用的比较研究
摘要
使用商业铜纳米粒子 (Cu-NPs)、铜/氧化亚铜纳米粒子 (Cu|Cu2O-NPs) 和硫酸铜 (CuSO4) 合成了纳米复合材料和基于聚(己二酸丁二醇酯 - 共对苯二甲酸酯)(PBAT)的复合材料,分别。 Cu|Cu2O-NPs 使用化学还原合成,并通过 X 射线衍射 (XRD) 和透射电子显微镜 (TEM) 进行表征。 Cu|Cu2O-NPs 的合成产生了 Cu 和 Cu2O 的混合物,其中金属 Cu 的球形形态的直径约为 40 nm,而 Cu2O 的直径为 150 nm。为了制备纳米复合材料 (NCs) 和复合材料 (MC),通过非原位方法将 NPs 和 CuSO4 盐以 1%、3% 和 5% p/p 的浓度掺入 PBAT 基质中。傅里叶变换红外光谱 (FTIR)、拉伸试验、差示扫描量热法 (DSC)、热重分析 (TGA) 和琼脂扩散测定法用于结构、热机械和抗菌表征。结果表明,增强材料没有改变 PBAT 的化学结构,只是略微增加了结晶百分比。 PBAT 的机械和热性能随着填料的加入没有太大变化,除了拉伸强度和热稳定性分别略有增加之外。琼脂扩散抗菌试验表明,NCs和MCs对非耐药菌株粪肠球菌具有良好的抑制作用。 , 变形链球菌 , 和金黄色葡萄球菌 .基于 CuSO4 的 MCs 具有最高的杀菌效果,甚至对耐药菌鲍曼不动杆菌 .
介绍
大多数塑料材料由化石燃料制成,实际上不可降解,这引起了人们对经济和环境可持续性的担忧 [1, 2]。因此,从其他来源开发和合成可生物降解材料已受到科学界的广泛关注,其目标是减少石油基塑料的产量 [3,4,5]。可生物降解的聚合物已开始在解决这些问题方面发挥重要作用,作为化石燃料的一种有前途的选择,以及一类被称为生物纳米复合材料的新型材料,通过纳米技术,这些材料具有更好的性能 [6,7,8,9 ,10]。
生物纳米复合材料由有机基质组成,其中分散有无机纳米材料 [8, 11,12,13]。无机成分的不同形态和尺寸,如纳米颗粒、纳米管、纳米片、纳米线和纳米粘土,对聚合物基体的性能有相当大的影响。由于无机组分和聚合物基体的表面积、高表面反应性、优异的热稳定性和高机械强度之间的协同作用,光学、热、机械、磁和光电性能得到改善 [14,15,16] .聚合物化学和微纳米制造技术的广泛创新推动了聚合物生物纳米复合材料的研究,不仅用于生产改进的结构,而且用于制备具有有趣特性和高度复杂应用的新功能材料 [17,18 ,19]。几种天然或合成来源的生物聚合物,如聚乳酸 (PLA) [20] 和聚对苯二甲酸丁二酯-己二酸酯-共聚对苯二甲酸酯 (PBAT),已被广泛研究 [21, 22]。
目前用作纳米复合材料基质的一种聚合物是 PBAT [23]。这种合成生物聚合物是基于聚合物链中的单体 1,4-丁二醇、己二酸和对苯二甲酸的线性脂肪族可生物降解聚酯 [24]。由于其高分子量和长支链分子结构,使其具有与低密度聚乙烯相似的特性,使其具有柔韧性 [24,25,26]。 PBAT 的主要限制是其机械强度差;然而,通过添加纳米级负载,可以克服这一缺点,从而赋予该材料多功能特性,例如更好的热机械性能[6, 27]。
目前,还迫切需要开发生物纳米复合材料,通过将具有已知抗菌活性的纳米颗粒掺入或增强聚合物基质已经具备的抗菌性能来控制或防止微生物定植。在后一种情况下,聚合物基质杀生物能力的显着提高与生物纳米复合材料的两种成分之间的协同作用有关 [28, 29]。因此,聚合物不仅为纳米颗粒提供了支撑基质,而且还可以提高抗菌性能并扩展生物纳米复合材料的可能应用,以满足生物医学应用或医疗器械(如气管导管、血管和导尿管)的各种要求[30]。 ,31,32]。然而,PBAT 在医疗器械中的使用尚未得到广泛研究;只有少数文章报道了其在一些临床应用中使用的可能性[1]。
一些研究报告了使用金属纳米颗粒作为抗菌剂。这些材料的内在生物学特性取决于多种因素,例如所涉及的金属、粒度、结构和表面积。这些因素的所有可能组合都可以延迟抗菌素耐药性 [33]。大多数纳米复合材料的抗菌研究都集中在食品包装上,而杀菌活性总是针对相同的细菌。不确定细菌是否会像对药物一样对杀生物纳米粒子产生抗药性。因此,这项工作的目标之一是评估含有 PBAT 和不同浓度 Cu-NPs 的纳米复合材料的抗菌活性,以用于制造牙科工具的潜在用途。此外,我们对基于 PBAT 的材料的热机械和抗菌性能进行了完整的比较研究。 PBAT 纳米复合材料是用三种不同浓度的铜纳米颗粒制备的。类似地,使用 Cu|Cu2O-NPs 作为负载制备纳米复合材料。最后,以与制备纳米复合材料相同的浓度制备了基于 CuSO4 的复合材料。评估了纳米复合材料和 PBAT 复合材料对金黄色葡萄球菌的杀生物活性 , 导致皮肤感染,如毛囊炎、疖病和结膜炎; 变形链球菌 ,部分负责牙菌斑和牙生物膜;和粪肠球菌 和鲍曼不动杆菌 ,这可能会导致危害人类的感染,尤其是在医院环境中。
材料和方法
材料
用于制备纳米复合材料的 PBAT(Ecoflex)由 BASF(德国路德维希港)提供。其分子结构见附加文件 1:图 S1(补充材料)。 99.99% 纯金属铜纳米粒子(Sigma-Aldrich, St. Louis, MO, USA)的直径在 100 到 200 纳米之间。对于 Cu|Cu2O-NPs 的合成,使用 CuSO4 作为前体,抗坏血酸 (C6H8O6) 作为还原剂,氢氧化钠 (NaOH) 作为 pH 控制剂。此外,CuSO4(Sigma-Aldrich)用于制备复合材料。
通过化学还原合成纳米颗粒
Khan等人提出的一种合成方法。 [34] 用于获得 Cu|Cu2O-NPs。合成开始时将 CuSO4 × 5H2O 溶解在蒸馏水中,得到 120 mL 0.1 M 的溶液。接着,将 120 mL CuSO4 加入浸入丙二醇浴的烧瓶中,然后快速加入 50 mL C6H8O6 溶液。将混合物以约390 rpm剧烈搅拌30 分钟,同时将温度升至80 ℃,然后滴加30 mL的NaOH溶液,并连续搅拌该溶液2 小时。使最终溶液静置过夜,然后除去上清液。将浓缩物离心并用蒸馏水和乙醇洗涤。最后,使用超声波设备将颗粒分散,置于培养皿中,在 60 °C 下烘干过夜(参见附加文件 1:图 S2)。
纳米复合材料合成
为了制备纳米复合材料和复合材料,将 Cu-NPs、Cu|Cu2O-NPs 和 CuSO4 盐以 1%、3% 和 5% 的浓度掺入 PBAT 基质中。首先,将 PBAT 熔化,然后在扭矩流变仪(型号 835205,Brabender GmbH &Co. KG,Duisburg,Germany)中以 60 rpm 和 140 °C 的工作温度混合 7 分钟(附加文件 1:图 S4)。最大负载为 5%,因为更高的负载会在拉曼光谱中产生荧光效应(附加文件 1:图 S3)。
特征化
对获得的纳米复合材料和复合材料进行表征,以研究它们与 PBAT 聚合物的差异。同样,我们研究了聚合物中不同浓度的Cu-NPs、Cu|Cu2O-NPs和CuSO4如何影响其力学、热、形态、结构和杀菌性能。
Cu-NPs 和 Cu|Cu2O-NPs 通过 X 射线衍射 (XRD) 和透射电子显微镜 (TEM) 进行表征。通过热重分析 (TGA) 表征了含有 Cu-NPs (NCs-PBAT/Cu) 和 Cu|Cu2O-NPs (NCs-PBAT/Cu|Cu2O) 的 PBAT 纳米复合材料和含有 CuSO4 的 PBAT 复合材料 (MCs-PBAT/CuSO4) 、差示扫描量热法 (DSC)、扫描电子显微镜 (SEM)、傅里叶变换红外光谱 (FTIR)、XRD、拉伸试验和使用琼脂扩散的抗菌活性测定。制备每种纳米复合材料的100-mm × 100-mm × 1-mm的板状样品,使得在每次分析中均质化的样品尺寸相同。为了获得板形状,NCs-PBAT/Cu、NCs-PBAT/Cu|Cu2O 和 MCs-PBAT/CuSO4 使用 Labtech 液压机(型号 LP-20B;Labtech Engineering Co., Ltd., Samutprakarn, Thailand ) 在 160 °C 和 110 巴下保持 5 分钟。预热和冷却时间分别为 15 min 和 1 min(附加文件 1:图 S4)。
形态和结构特性
为了验证纳米颗粒的纳米尺度以及合成的粉末是Cu和Cu2O纳米颗粒的混合物,使用XRD进行结构分析,并使用TEM进行形态分析。
使用 JEM 1200 EX II 透射电子显微镜(JEOL, Ltd., Tokyo, Japan)在 120 kV 电压下获得 Cu|Cu2O-NPs 的 TEM 显微照片。通过将一滴在乙醇中稀释的纳米颗粒放在 200 目碳涂层铜网上来制备样品。此外,还通过电子衍射图分析了纳米颗粒。
Cu-NPs、Cu|Cu2O-NPs、纳米复合材料和复合材料的 XRD 光谱是使用 Bruker Endeavor 衍射仪(D4/MAX-B 型;Bruker,Billerica,MA,USA)获得的。 2θ 的扫描范围为 4 到 80°,步长为 0.02°,计数时间为 1 s。衍射仪使用铜阴极灯(λ =1.541 Å).
使用具有衰减全反射 (ATR) 功能的 Spectrum Two FTIR 光谱仪 (× 1720) (PerkinElmer, Waltham, MA, USA) 获得纳米复合材料的 FTIR 光谱。每个光谱是通过在 4000-500 cm -1 范围内连续扫描获得的 分辨率为 1 cm −1 .
机械性能(拉伸试验)
拉伸测试基于 ASTM D638 标准,在 smarTens 万能试验机(005 型号;Emmeram Karg Industrietechnik,Krailling,德国)上以 50 mm/min 的测试速度和 1 kN 的称重传感器进行。 V 型试样是在 160 °C 的成型温度下通过压缩制造的。预热、压制和冷却时间分别为 7、5 和 1 min。所研究的每种NC和MC分别制作了5个样品,并获得了拉伸强度、极限伸长率和模量。
热性能
TGA 使用 TG 209 FI Iris® 热微量天平(NETZSCH-Gerätebau GmbH,Selb,德国)进行。将 3 至 10 mg 的样品置于铝坩埚中,然后将其装入仪器中。通过在 N2 气氛下以 10°C/min 的速率将样品从 20°C 加热到 600°C,测量作为温度函数的质量变化。
使用 NETZSCH 差示扫描量热仪(DSC 204 F1 型号)进行 DSC 分析。将纳米复合材料样品(5-10 mg)置于密封的铝坩埚中,在 20 mL/min 的恒定 N2 流速下,以 10 °C/min 的速率将其从 25 加热到 200 °C。熔化温度 (T m) 是从该 DSC 分析中获得的。
使用琼脂扩散法测定 NC 和 MC 的抗菌活性
使用在琼脂中的扩散生长动力学方法测定基于 Cu-NPs、Cu|Cu2O-NPs 和 CuSO4 的纳米复合材料和复合材料的抗菌活性。分析按照 Jaramillo 等人的协议分两个阶段进行。 [35]。使用了四种细菌菌株:两种临床菌株,A.鲍曼不动杆菌 (ABA 538) 从院内感染和大肠杆菌中分离出来。粪便 (6.4) 来自口腔感染和两个收集菌株 S.金黄色葡萄球菌 (ATCC) 和 S。变种人 (ATCC 25175).
第一阶段包括抗菌活性的定性评估,以选择使用三种浓度的纳米复合材料和复合材料中的哪一种来进行定量测试,以减少实验设计,因为使用三种负载浓度将非常昂贵。在评估测试之后,选择具有显示最佳接触抑制的负载百分比的样品。为了进行定性测试,A.鲍曼不动杆菌 (ABA 538),E。粪便 (6.4),S。金黄色葡萄球菌 (ATCC) 和 S。变种人 (ATCC 25175) 分别接种在胰蛋白酶大豆琼脂 (TSA) 上,并在 37°C 下孵育过夜。培养后,选择一个分离良好的菌落,并使用接种环将其转移到含有 4-5 mL TSA 肉汤的管中。将肉汤再次在 37°C 下孵育过夜,直到达到或超过 McFarland 标度的 0.5 浊度。然后使用浊度计用盐水溶液将接种物的浊度调节至麦克法兰标度上的 0.5。制备的悬浮液含有约 1 × 10 8 CFU/mL,按1:10稀释至终接种浓度为10 7 CFU/毫升。用每个接种物均匀地接种 TSA 平板。然后,张(10 × 10 mm 2 ) 的纳米复合材料和复合材料,浓度为 1%、3% 和 5%,加上 PBAT 对照,被放置在 TSA 板的表面上并检查以确保它们粘附良好。最后将平板置于烘箱中,37 ℃培养24 h,观察对PBAT样品的抑制作用。
生长动力学方法的第二阶段包括仅对那些在定性测试中明显接触抑制的纳米复合材料和复合材料进行的定量测试。为保持无菌状态,使用 1200 系列 A2 型生物安全柜(ThermoFisher Scientific, Waltham, MA, USA)进行测试。首先,将样品置于无菌培养皿中并将它们带到生物安全柜中,在那里将它们每侧暴露在紫外线下 15 分钟进行预处理。接下来,将每个菌株的 24 小时细菌培养物调整到 McFarland 标度上的 0.5 浊度,以随后创建六个系列稀释(1、2、3、4、5 和 6)。对稀释度 4、5 和 6(一式三份)进行初始计数以确定零时的计数。
湿室,每个评估时间(2、4、6 和 8 小时)和每个菌株一个,通过将用无菌蒸馏水润湿的无菌纱布放入无菌培养皿中来制备。然后,将无菌载玻片放置在每个湿室中,使得上侧不接触湿纱布。接下来,三个 1 × 1-cm 2 在无菌夹子的帮助下,将纳米复合材料和复合材料片材以及作为对照的 PBAT 片材放置在腔室中。将稀释液(20 μL)沉积在每个方片上,并将小室在37 °C下孵育2、4、6和8 h。
孵育后,提取湿室,并将每个聚合物片沉积在装有 1 mL 无菌蒸馏水的 Falcon 管内。将管涡旋 2-5 分钟 [35]。从 Falcon 管中的产品制成三个稀释液。含有 TSA 的培养皿被分成四个部分。将大约三到五滴(对应于 20 μL)三种稀释液中的每一种和一滴未稀释的 Falcon 管内容物放入象限中。琼脂平板必须完全干燥,以便几乎立即吸收液滴。然后将板在 37°C 下孵育 24 小时,然后用菌落计数器进行菌落计数。获得的数据乘以所使用的稀释因子,并使用对数函数或存活百分比绘制在图表中。
结果与讨论
流变测量法用于在接近处理纳米复合材料的实际条件的条件下获得纳米复合材料的流变特性的动态测量值。为此,进行了测量以控制熔体混合期间粘度的变化。这些测量的结果显示在附加文件 1:图 S5 中。电机扭矩的增加与聚合物的熔融粘度有关 [21, 36],在混合 4 min 后,这些值开始保持不变。这证实了在这项工作中建立的7 min的混合时间足以实现完全混合。
PBAT 和 NCs-PBAT/Cu 1% 基质的扭矩值约为 19.86 N m。曲线(附加文件 1:图 S5)表明,1% 浓度的 Cu-NPs 对基体的机械性能几乎没有影响,但 NCs-PBAT/Cu 3 获得了较低的平衡扭矩值 18.4 和 17.4 N m % 和 NCs-PBAT/Cu 5%,分别。这些结果清楚地表明,NCs-PBAT/Cu 的可加工性相对于 PBAT 基质得到了改善 [37]。使用 NCs-PBAT/Cu|Cu2O 的混合物获得了类似的结果,其中平衡扭矩值随着负载百分比增加到 3% 而降低,但 5% 负载产生的值非常接近 1% 负载的值。 Cu|Cu2O-NPs。对于 1%、3% 和 5%,平衡扭矩值分别为 19.39、19.07 和 19.37 Nm。对于 MCs-PBAT/CuSO4 混合物,平衡扭矩值随着 CuSO4 负载的增加而增加,即 18.71 N m 为 1%,19.16 N m 为 3%,19.79 N m 为 5% 负载。这种行为可归因于 CuSO4 晶体的尺寸。同时,附加文件 1:图 S5 显示所有纳米复合材料和复合材料的平衡扭矩随着混合时间的增加而稳定,表明混合器中没有发生热分解,可能是因为纳米颗粒降低了聚合物链和复合材料之间的内聚力。最有可能在混合过程中进行自润滑[37]。
形态和结构特性
首先,分析了通过化学还原获得的纳米颗粒。 Cu|Cu2O-NPs 的合成结果如图 1b 所示。 TEM 显微照片显示球形颗粒和多面体颗粒的混合物。球形纳米粒子的平均直径为 26 nm(图 1c),而多面体纳米粒子的直径介于 80 和 160 nm 之间。这些纳米粒子的组成是通过选区电子衍射 (SAED) 确定的(图 1c),它发现了对应于金属 Cu 和 Cu2O 的相。图 1a 中所示的衍射图证实了这一发现。在2θ =36.3°、42.17°、43.42°、50.63°、61.47°和74.37°处清楚地观察到六个衍射峰。由于纳米颗粒是通过将 CuSO4 化学还原为 CuO 来合成的,因此衍射峰由 X'Pert HighScore X 射线粉末衍射图数据库中的 Cu 数据验证。我们观察到2θ =43.2°、50.63°和74.37°处的峰属于金属Cu衍射面(111)、(200)和(220)。其他三个峰表明合成的纳米粒子含有不止一种物质,因此衍射图是两者的结合。 Wijesundera [38] 使用 XRD 分析了 Cu2O 薄膜,并表明在 2θ =36.3°、42.17° 和 61.47° 处衍射的平面对应于米勒指数 (111)、(200) 和 (220)。根据SAED分析结果,这些指标属于面心立方结构(FCC),对应于抗萤石结构中心区域的一部分,与Cu2O的结构一致。
<图片>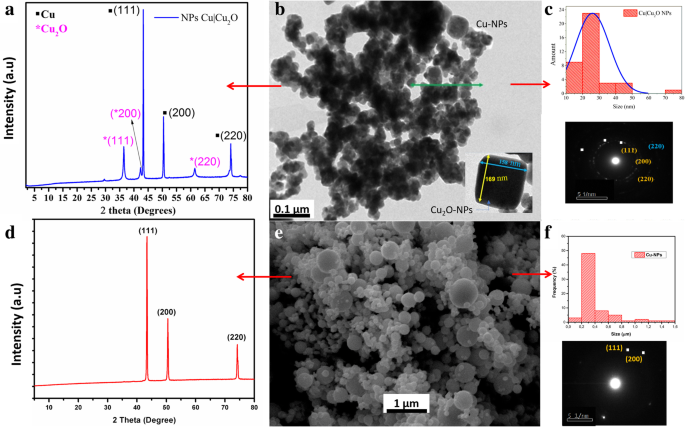
一 合成的 Cu 和 CuO2 纳米粒子的 XRD。 b , c 合成纳米颗粒的 TEM 图像、尺寸分布和衍射图。 d Cu纳米颗粒的XRD。 e , f Cu纳米颗粒的TEM图像、尺寸分布和衍射图
王等人。 [39] 发现在通过化学还原合成 Cu-NPs 的过程中,颗粒的大小在 100 到 150 纳米之间。他们使用 C6H8O6 作为还原剂,使用聚乙烯吡咯烷酮 (PVP) 作为表面活性剂。表面与 Cu2O 的表面不对应,因为 PVP 有助于稳定生长中的种子,从而避免它们的氧化。然而,我们研究的目的是合成Cu2O NPs,这可以通过化学还原而不使用稳定剂如PVP 来实现。
用于制备纳米复合材料的 Cu-NPs 是球形的,直径在 100 到 200 纳米之间(图 1e,f)。在图 1d 所示的 Cu-NP 的 XRD 图中,在 43.60°、50.72° 和 73.95° 处清楚地观察到的三个峰分别对应于晶面(111)、(200)和(220)。具有Fm3m空间群的立方晶体结构(JCPDS No.85-1326)[55]与SAED分析发现的结构一致(图1d)。
据供应商称,我们研究中使用的金属颗粒是通过机械研磨系统获得的。这种方法的缺点是一小部分粒子 (~ 10%) 大于 500 nm。然而,这并没有对我们的调查目标产生负面影响。下面,我们将展示这种分散体如何影响 PBAT 基体的热机械性能。重要的是,机械研磨方法不使用前体或稳定剂,就像湿法合成方法一样,称为化学还原方法。因此,通过研磨获得的 Cu-NPs 的表面不会被来自稳定剂或反应副产物的分子吸附钝化。因此,这些 Cu-NPs 虽然不会显着改善聚合物的机械性能,但也不会降解它们。然而,由于Cu 2+ 的迁移,必须提高抗菌性能。 在非钝化表面上促进。
图 2 显示了 NCs-PBAT/Cu(图 2a)、NCs-PBAT/Cu|Cu2O(图 2b)和 MCs-PBAT/CuSO4(图 2c)的 XRD 谱。图 2c 以三种浓度(1、2 和 3% w /w )。将这些衍射图与 PBAT 聚合物基质的衍射图进行比较,以证明载荷对聚合物结构的影响。 PBAT 衍射图显示在 2θ =16.1°、17.3°、20.2°、23.1° 和 25° 处具有五个衍射峰的衍射图案,对应于平面 (011)、(010)、(101)、(100) 和(111),分别。该分析揭示了聚合物基质中存在结晶性。 Arruda 等人对 PBAT 的表征。 [40] 使用 XRD 还发现了与本研究中发现的相同角度的五个衍射峰,对应于相同的平面。
<图片>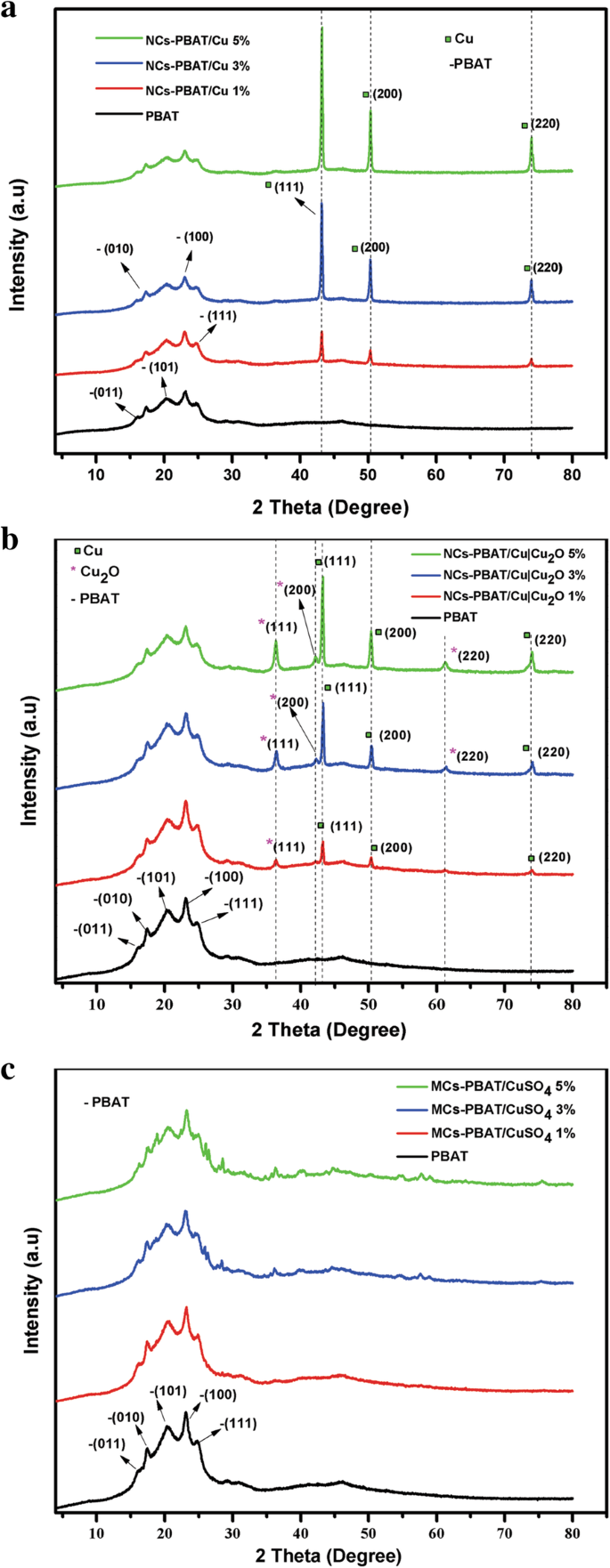
PBAT、NCs-PBAT/Cu、NCs-PBAT/Cu|Cu2O和MCs-PBAT/CuSO4的衍射图
负载有 Cu-NPs 的纳米复合材料的衍射图如图 2a 所示。 43°、50°和74°的2θ信号是具有Fm3m空间群的Cu的FCC结构的平面(111)、(200)和(220)的特征(JCPDS No.85-1326) [ 41]。在 NCs-PBAT/Cu 的衍射图中没有观察到对应于 CuO 或 Cu2O 的相,因此我们得出结论,纳米颗粒在纳米复合材料的合成过程中没有被氧化。此外,衍射图表明纳米颗粒不会影响或改变 PBAT 的结构,并且峰的强度与 Cu-NP 的负载百分比成正比。 NCs-PBAT/Cu|Cu2O 的衍射图在 2θ =36.4°、43°、42.4°、50°、61.5° 和 74° 处有六个特征峰(图 2b)。根据文献和纳米粒子的分析,只有三个对应于金属Cu,36.4°、42.4°和61.5°的峰属于Cu2O,根据图1a所示的这类纳米粒子的光谱[35 ].
随着基体内浓度的增加,对应于 Cu|Cu2O-NPs 增强体的衍射峰变得更加强烈,但随着负载的加入,属于聚合物结晶区的峰强度略有下降。奇夫拉克等人。 [42] 在 PBAT 中使用纳米粘土负载的研究中报告了类似的结果。他们认为负载-聚合物界面没有明显的结晶性,因此聚合物的晶体结构没有变化。然而,随着基质中负载浓度的增加,PBAT 衍射峰强度的降低表明 PBAT 的结晶度下降。因此,负载阻碍了 PBAT 的晶体生长。这可以解释随着Cu|Cu2O-NPs的增加,属于PBAT的衍射峰略有减少。
图 2c 显示了 1%、3% 和 5% 三种浓度的 CuSO4 的 MC-PBAT/CuSO4 的 XRD 谱。添加 1% CuSO4 负载不会导致聚合物发生变化。 3% 和 5% CuSO4 负载曲线仅显示 2θ =36.4°、40.25°、43.94°、57.9° 和 75.7° 处的峰强度增加最小,这属于存在的 Cu 和 Cu2O,表明在混合过程中,部分 Cu2SO4 被还原和氧化。至于 PBAT 的结晶区,CuSO4 增强剂浓度的增加降低了 PBAT 中衍射峰的强度,就像 NCs-PBAT/Cu 和 NCs-PBAT/Cu|Cu2O 一样。因此,在聚合物基体中加入 CuSO4 会降低其结晶能力,这可能是因为 CuSO4 阻碍了微晶的生长。由于没有报道复合材料中 CuSO4 XRD 光谱的其他信息,我们将不得不研究其在可生物降解聚合物中的行为。基体结晶度计算公式为:
$$ {X}_{\mathrm{c}}=\frac{I_{\mathrm{c}}}{I_{\mathrm{c}}+{I}_{\mathrm{a}}} $$ (1)其中我 c 是晶相峰面积,I c + I a 是衍射图下的总面积。表 1 中给出了每种材料的结晶度值。这些结果表明,随着 PBAT 基体中 Cu-NPs 和 Cu|Cu2O-NPs 浓度的增加,结晶度的百分比增加,这随着各衍射图中峰的强度。
另一方面,衍射图表明纳米颗粒不会影响或改变 PBAT 的结构,并且峰的强度与 Cu-NPs 和 Cu|Cu2O-NPs 的负载百分比成正比。此外,与纯态聚合物相比,CuSO 4 前体盐的加入降低了聚合物的结晶度。发生这种情况是因为在纳米复合材料中添加负载浓度增加了结晶度的相对百分比,但降低了 PBAT 的结晶度,结果通常被报告为总结晶度百分比略有增加。 MCs-PBAT/CuSO4 负载在其 XRD 光谱中没有出现结晶峰。因此,它们对结晶度的增加没有贡献,而是导致聚合物链中结晶度的降低,这解释了复合材料中结晶度总百分比的降低。一些研究表明,金属纳米粒子在聚合物链的取向中充当成核中心,进而提高聚合物的结晶度[43]。
FTIR(附加文件 1:图 S6)光谱显示不同负载浓度下的特征峰频率相同但强度不同。光谱表明,随着聚合物基质中纳米颗粒浓度的增加,对应于 NCs-PBAT/Cu 和 NCs-PBAT/Cu|Cu2O 的峰强度相对于 PBAT 增加。因此,PBAT 的链和纳米颗粒之间没有有效的相互作用。 Had there been interaction, some of the signals in the FTIR spectrum would have been displaced as a result of the interaction of the functional groups of the polymer with the surface of the PBAT [40].
Mechanical Properties (Tensile Test)
To give multifunctionality to biopolymers, nanomaterials that provide special properties to a nanocomposite are usually incorporated. Their inclusion will change the mechanical properties of the material and the intensity of the changes is directly related to the union of the nanostructure with the polymer network [44]. We conducted tensile tests on the nanocomposites and the composite material. The tensile strength and maximum deformation values are summarized in Table 2.
Figure 3 shows the average curves of the tensile tests on the nanocomposites and composite material. As the permanent deformation of the material began, the effect of the concentration of the nanoparticles in the polymer could be distinguished. Figure 3a shows the results for NCs-PBAT/Cu. The results show that the inclusion of nanostructures did not considerably affect the elastic range but there were noticeable changes in the yield strength. As the concentration of the Cu-NPs increased, maximum resistance increased and maximum elongation decreased. These changes clearly indicate that the nanostructures harden the PBAT. At 3% concentration of Cu-NPs, the tensile strength slightly increased but the elongation percentage in the fracture decreased between 30 and 35%.
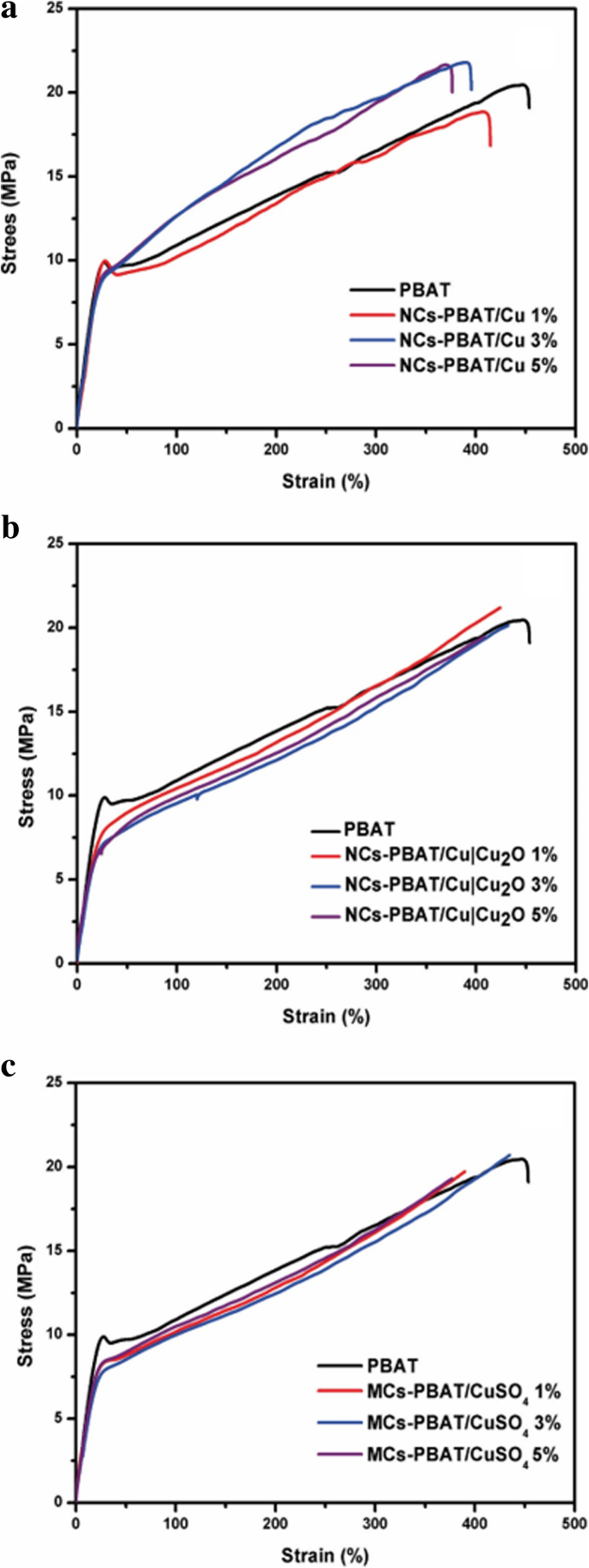
Stress and strain of PBAT, NCs-PBAT/Cu, NCs-PBAT/Cu|Cu2O, and MCs-PBAT/CuSO4
Figure 3b shows the results of the tensile tests on the NCs-PBAT/Cu|Cu2O. The 1% load nanocomposite clearly showed an increase in tensile strength and elongation with respect to the PBAT. There was no appreciable effect on the elastic range, but it did appear to be above the yield stress. In addition, the curve for the 3% load NCs-PBAT/Cu|Cu2O shows there was no significant difference with respect to the PBAT. The same behavior is seen with curve for the 5% load NCs-PBAT/Cu|Cu2O. The curves for MCs-PBAT/CuSO4 (Fig. 3c) show that the yield stress decreased for the three concentrations of CuSO4 with respect to the PBAT.
From the results, we can conclude that the reinforcements did not significantly change the mechanical properties of the PBAT. Venkatesan and Rajeswari [45] showed a significant increase in mechanical properties by incorporating ZnO nanoparticles in a PBAT matrix with respect to that of the PBAT. Similar results with some improvements were obtained by Chen and Yang [46]. They elaborated a PBAT nanocomposite with montmorillonite nanoparticles using melt blending.
Our investigation found that the NCs-PBAT/Cu|Cu2O 3 and 5% and MCs-PBAT/CuSO4 1 and 5% had slightly decreased tensile strength, that is, there were no significant variations in the mechanical properties. However, the NCs-PBAT/Cu|Cu2O 1% and MCs-PBAT/CuSO4 3% had slightly increased tensile strength. Therefore, no reinforcement at any concentration in the matrix caused remarkable variations in the mechanical properties of the PBAT. In addition, as the concentration of Cu-NPs increased, their mechanical properties increased the resistance of the PBAT but elongation could not be maintained. The results of the tensile tests showed that the commercial Cu nanoparticles improved the tensile strength of the PBAT slightly more than did the Cu|Cu2O nanoparticles and the CuSO4 particles. The difference between the tensile properties found in our investigation and those in the literature could be attributed to load dispersion because the agglomerated particles act as stress concentrators [47]. Finally, the variations in the test values were explained by the preparation conditions of the test samples, the degree of crystallinity of the PBAT, the molecular mass, the degree of interaction at the polymer-reinforcement interface, and the load dispersion because the agglomerates in the matrix could act as stress concentrators.
Thermal Properties
One of the disadvantages of the PBAT is its low thermal stability because the fusion process can degrade its polymer chains [48]. Therefore, the effect of nanometric and micrometric loads on the decomposition of this biopolymer must be investigated. TGA of NCs-PBAT/Cu, NCs-PBAT/Cu|Cu2O, and MCs-PBAT/CuSO4 was carried out to observe the changes in the thermal stability of the PBAT caused by the presence of Cu nanoparticles in the matrix. The TGA results are shown in Fig. 4, and the initial (T di) and final (T df) decomposition temperatures of the analyzed samples are summarized in Table 3. The thermograms show that the polymer without any load had a weight loss of 1% at 420.77 °C, while the nanocomposites NCs-PBAT/Cu 1, 3, and 5% presented a weight loss of around 3% (Fig. 4a). This suggests that the presence of Cu-NPs at concentrations of 3 and 5% slightly increases the thermal stability of the nanocomposites compared to that of the unloaded polymer. After the final thermal decomposition, the degradation percentages, at around 420–427 °C, of the PBAT matrix and nanocomposites NCs-PBAT/Cu 1, 3, and 5% were 98.9, 97.5, 95.4, and 96.8%, respectively. The residues were higher for Cu-NPs-incorporated nanocomposite samples. Similar results have been reported for PBAT nanocomposites with different loads of Ag-NPs [49].
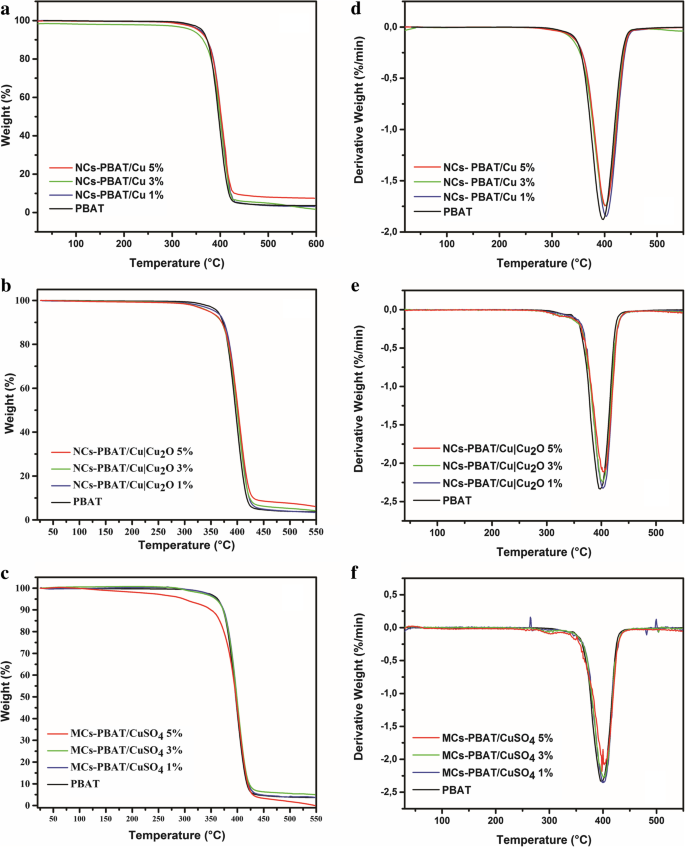
TGA of a PBAT and NCs-PBAT/Cu, b NCs-PBAT/Cu|Cu2O, and c MCs-PBAT/CuSO4, DTG of d PBAT and NCs-PBAT/Cu, e NCs-PBAT/Cu|Cu2O, f MCs-PBAT/CuSO4
Although no significant change is seen among the curves in Fig. 4b for the NCs-PBAT/Cu|Cu2O, the results show that as the Cu|Cu2O-NPs increased in the polymer structure, T di increased and T df decreased with respect to the initial and final degradation temperatures of PBAT; in addition, the total mass loss decreased. By calculating the derivative of the mass with respect to the temperature, we obtained the curves in Fig. 4d–f for the indicated peaks of the nanocomposite with Cu|Cu2O-NPs and found that T df, at which the maximum decomposition occurs, was between 402 and 403 °C (Table 3).
The CuSO4 loads incorporated into the polymer matrix, i.e., MCs-PBAT/CuSO4, yielded the same behavior as that of the NCs-PBAT/Cu|Cu2O, with an increase in T di and a decrease in T df with respect to the PBAT polymer. T di values of the NCs-PBAT/Cu|Cu2O and the MCs-PBAT/CuSO4 were greater than that of the NCs-PBAT/Cu, but the T df and degradation percentage values were less than those of the nanocomposites with Cu-NPs loads.
This enhancement of the thermal stability of the PBAT is attributed to the barrier effect of the loads. The loads were also supposed to have a shielding effect on the matrix to slow the rate of mass loss of the decomposition product [50]. The data obtained by our analysis were compared with published results to verify that the indicated behavior is usual for this type of polymer. Sinha Ray et al. [51] found by thermal analysis of PBAT reinforced with nanoclays that the degradation temperatures of the nanocomposites were greater than or at least equal to that of the PBAT. In general, the reinforcements improve the thermal stability of the polymer matrix because they act as a heat barrier, which improves the total thermal stability of the system. However, the studies of Sinha Ray et al. and this investigation showed that the thermal stability of the nanocomposite and PBAT compounds only slightly improved. To explain the relatively low improvement in the thermal stability of some nanocomposites, Sinha Ray et al. assumed that in the early stages of thermal decomposition, the reinforcements displace the decomposition to higher temperatures, but in a second stage, the clay layers accumulate heat and then act as a source of heat. This heat source, along with the heat flow supplied by the external heat source, promotes the acceleration of decomposition. This could explain the behavior of the reinforcements in the NCs-PBAT/Cu|Cu2O and MCs-PBAT/CuSO4. Thus, we conclude that the thermal properties of the nanocomposites and the composite material slightly improve but not significantly. On the other hand, the results of DSC (Additional file 1:Figure S7 and Table S1) indicated that the addition of reinforcements to the matrix slightly hindered the kinetics and degree of crystallization of the PBAT. The addition of clays increased the crystallization temperature from 1 to 10 °C and the melting temperature from 1 to 5 °C. These phenomena were probably due to an increase in the viscosity of the polymer with the addition of clays, which reduced the mobility of the macromolecular chains against the growth of crystals.
Comparative Evaluation of the Antimicrobial Activity of NCs-PBAT/Cu, NCs-PBAT/Cu|Cu2O, and MCs-PBAT/CuSO4
Qualitative Test
After the experimental procedure was performed, we wanted to observe whether bacterial colonies were inhibited by each PBAT sample, i.e., NCs-PBAT/Cu 1, 3, and 5%; NCs-PBAT/Cu|Cu2O 1, 3, and 5%; and MCs-PBAT/CuSO4 1, 3, and 5%. We decided to use the 3% concentrations because the 1% concentrations did not produce enough bacterial inhibition and the 5% concentration produced behavior similar to that of the 3% concentration, the minimum percentage with activity that avoided toxicity in the polymer.
Quantitative Test
The study was carried out at different contact times using four bacterial strains and the PBAT samples NCs-PBAT/Cu 3%, NCs-PBAT/Cu|Cu2O 3%, and MCs-PBAT/CuSO4 3%. The times and colony-forming unit counts (CFU/mL) are presented in Table 4, and the bacterial activity and colony count for each Petri dish are shown in Fig. 5. In addition, a graphical analysis is shown in Fig. 6, where images of bacterial growth are also presented. The statistical analysis of the data is summarized in Table 5.
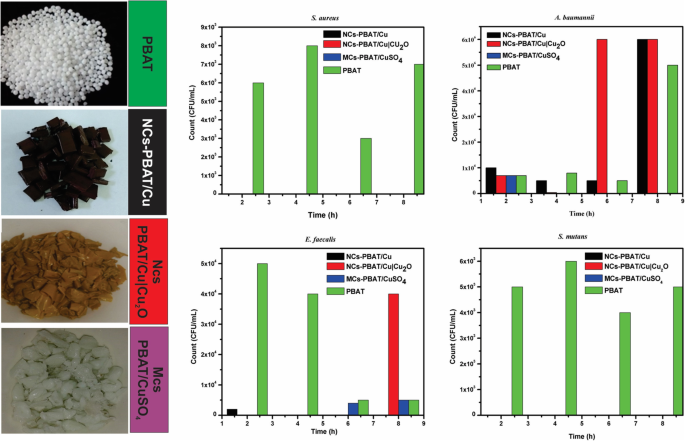
Bacterial activity and colonization count PBAT, NCs-PBAT/Cu-3%, NCs-PBAT/Cu|Cu2O 3%, and MCs-PBAT/CuSO4 3% for each strain of bacteria. 金黄色葡萄球菌 , Acinetobacter baumanni , Enterococcus faecalis , Streptococcus mutans
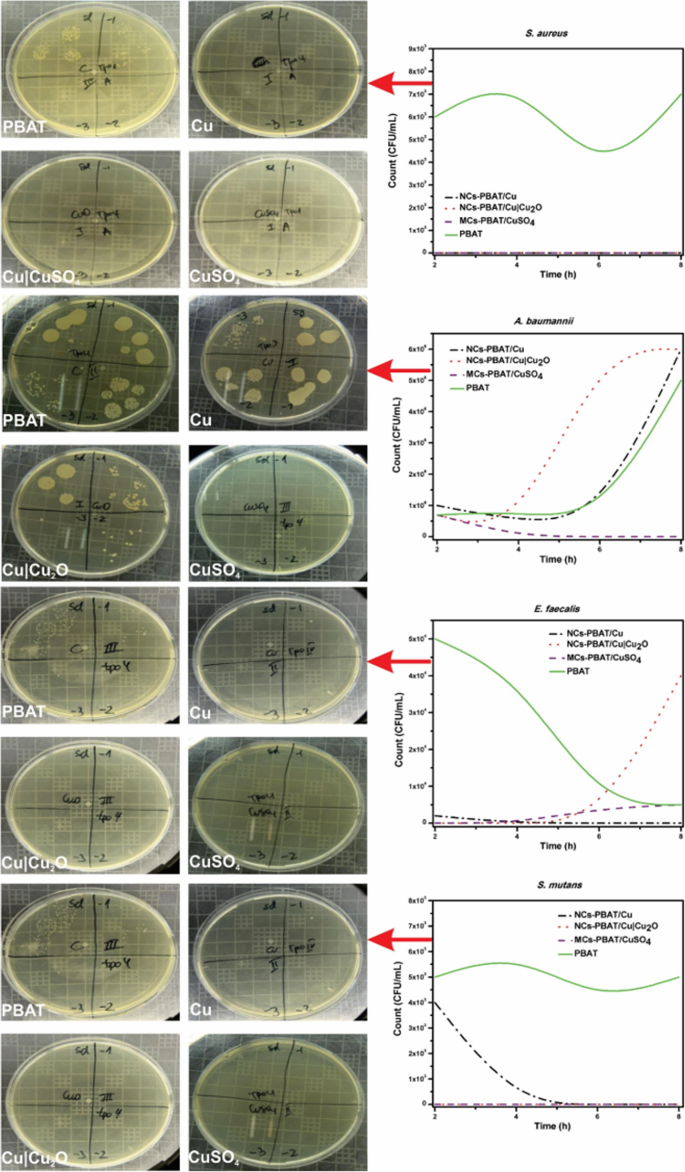
Graphical analysis of colony count (CFU/mL) vs time (h) of PBAT, NCs-PBAT/Cu-3% NCs-PBAT/Cu|Cu2O 3%, and MCs-PBAT/CuSO4 3% for each strain of bacteria. Enterococcus faecalis , Acinetobacter baumanni , Streptococcus mutans , Staphylococcus au reus
The study of A. baumannii found that the colonies grew in all periods (2, 4, 6, and 8 h) in the samples containing Cu-NPs, Cu|Cu2O-NPs, and PBAT. High bactericidal activity occurred with the sample containing CuSO4 during exposure times of 4, 6, and 8 h, decreasing from 7 × 10 5 to 0 CFU/mL. The sample containing Cu-NPs showed a significant increase in the growth of bacterial colonies from 1 × 10 5 to 6 × 10 6 CFU/mL, with an average of 2 × 10 6 CFU/毫升。 The bacterial colonies in the sample containing Cu|Cu2O-NPs grew from 7 × 10 5 in time I to 6 × 10 6 in time IV, with an average growth of 3.19 × 10 6 CFU/毫升。 Bacterial growth in the PBAT reached an average of 1.75 × 10 6 CFU/mL.
The study of E. faecalis found good bactericidal activity by the samples containing Cu-NPs, Cu|Cu2O-NPs, and CuSO4, with average colony growth of 5 × 10 2 , 1 × 10 4 , and 2.2 × 10 3 CFU/mL, respectively, while the PBAT did not show bactericidal activity and the colonies grew at all times. Colony growth in the sample containing Cu-NPs was 2 × 10 3 CFU/mL at 2 h then dropped to zero at 4, 6, and 8 h, whereas the samples containing Cu|Cu2O-NPs had 0 CFU/mL at times I, II, and III, but 4 × 10 4 CFU/mL at time IV. Samples containing CuSO4 prevented the growth of bacteria in times I and II with growth activity of 0 CFU/mL, but colonies grew to 4 × 10 3 and 5 × 10 3 CFU/mL for times III and IV, respectively. PBAT did not show bactericidal activity against E. faecalis .
The study of S. mutans found no colony growth in the samples containing Cu|Cu2O-NPs and CuSO4. The sample containing Cu-NPs showed very good bactericidal activity except at time I, at which colony growth was 4 × 10 3 CFU/mL, making the average growth for the four times 8 × 10 2 CFU/毫升。 PBAT without reinforcement showed no bactericidal activity against S. mutans . The samples containing Cu-NPs, Cu|Cu2O-NPs, and CuSO4 in contact with S.金黄色葡萄球菌 showed an excellent bactericidal response. They completely inhibited the growth of colonies, while PBAT did not show any bactericidal activity against S.金黄色葡萄球菌 , which grew an average of 6 × 10 3 CFU/mL.
In general, the antibacterial effectiveness of polymer-and-metal nanocomposites improves with a high surface/volume ratio, which increases the number of ions released from the nanoparticles into the polymer. The mechanism of the corrosion of Cu in aqueous solutions and the resulting Cu species vary with pH. In general, the species Cu2O and CuO are formed and can be dissolved in Cu ions. Elemental metal particles require the presence of water and oxygen molecules to release a small amount of ions. Therefore, retention of water and oxygen within the polymer is crucial for the release of Cu ions. Some properties of polymer-and-metal nanocomposites such as the crystallinity and polarity of the matrix, which constitute a barrier for the diffusion of water molecules and ions during their propagation, can affect the rate of release. Shankar and Rhim [49] prepared films composed of PBAT and Ag nanoparticles (PBAT/Ag-NPs) that showed strong antibacterial activity against E.大肠杆菌 和单核细胞增生李斯特菌 compared with that of PBAT films without Ag-NPs. Similar results were obtained by Venkatesan and Rajeswari [45] when they evaluated the antimicrobial activity of ZnO-NPs incorporated in a PBAT matrix. The PBAT compound, which was used as a control matrix, showed no antimicrobial activity compared to the PBAT/ZnO-NPs nanocomposite films. The results showed that the films had high bactericidal activity against the pathogens tested (E. coli 和S。金黄色葡萄球菌 ), with increased inhibition of bacterial growth as the ZnO load concentration increased from 1 to 10% by weight. This ability of Cu, Zn, and Ag nanoparticles to inhibit bacterial growth is mainly due to the irreparable damage to the membrane of the bacterial cells caused by the interaction between the surface of the bacteria and these oxides and metals [52, 53]. Compared with the works discussed above, our investigation found significant antimicrobial activity against inpatient and oral-resistant strains.
To complement this investigation, we performed water absorption tests using three different media and following point 7.4, “Long-Term Immersion”, in ASTM D570-98. The results of these tests are reported in the supplementary material, Additional file 1:Table S2–S4 and Figure S8, with their respective analysis. Analysis showed that sulfate-based composite materials absorb large amounts of water, even in acidic and basic environments. This phenomenon greatly affects the mechanical properties of these materials; however, resistant bacteria, such as A. baumannii , require an immediate Cu + distribution to control them. This explains the antimicrobial power of CuSO4 within the PBAT matrix.
结论
Using XRD and TEM, we determined that the synthesis of nanocomposites and material composites based on PBAT using chemical reduction and a mixture of metal Cu nanoparticles with CuO2, where Cu had a spherical morphology and Cu2O had a polyhedral morphology. The structural characterization of the NCs and MCs by FTIR and XRD showed that the Cu-NPs, Cu|Cu2O-NPs, and CuSO4 reinforcements did not modify the structure of the PBAT. However, they did slightly alter the percentage of its crystallinity, which increased with NPs and decreased with CuSO4. On the other hand, the mechanical properties of the PBAT for both the NCs and MCs did not vary significantly with the addition of reinforcements, meaning that the PBAT maintained its mechanical properties. From the thermal tests, we concluded that reinforcing the PBAT did not fundamentally improve its thermal properties, it only increased its thermal stability a few degrees Celsius, which is not significant. Antimicrobial analyses showed that the Cu|Cu2O-NPs within the PBAT generated antibacterial activity against E. faecalis 和S。 mutans and excellent bactericidal properties against S.金黄色葡萄球菌 . CuSO4 had a good bactericidal response against A. baumannii ,E。 faecalis , and S mutans and an exceptional response against S.金黄色葡萄球菌 . The PBAT without loads did not present bactericidal properties when in contact with the bacterial strains. In general, the addition of loads into the PBAT generates bactericidal activity that the polymer does not possess by itself. The addition of CuSO4 yielded the best antimicrobial response against the four strains used in this investigation. In the search for new applications for bionanocomposites, it will be essential to evaluate their antimicrobial response in food containers, medical devices, packaging, and other products; analyze their biocidal effects against other bacteria against which only NPs have antibacterial characteristics; and justify the expense associated with their synthesis.
缩写
- Cu|Cu2O-NPs:
-
Copper/cuprous oxide nanoparticles
- Cu-NPs:
-
Copper nanoparticles
- CuSO4 :
-
Copper sulfate
- DSC:
-
Differential scanning calorimetry
- FTIR:
-
傅里叶变换红外光谱
- MC:
-
Composite material
- MCs-PBAT/CuSO4 :
-
Composite materials of poly(butylene adipate-co-terephthalate) with copper sulfate
- NCs:
-
Nanocomposites
- NCs-PBAT/Cu:
-
Nanocomposites of poly(butylene adipate-co-terephthalate) with copper nanoparticles
- NCs-PBAT/Cu|Cu2O:
-
Nanocomposites of poly(butylene adipate-co-terephthalate) with copper/cuprous oxide nanoparticles
- PBAT:
-
Poly(butylene adipate-co-terephthalate)
- TEM:
-
透射电子显微镜
- TGA:
-
热重分析
- XRD:
-
X射线衍射
纳米材料
- 钴掺杂 FeMn2O4 尖晶石纳米粒子的制备和磁性
- 接触非平衡等离子体对 Mn Х Fe3 − X О4 尖晶石结构和磁性能的影响
- 基于苯基三甲氧基硅烷改性氧化铝纳米颗粒的 Al2O3:SiOC 纳米复合材料的形成和发光特性
- 富勒烯衍生纳米材料及其聚合物复合材料的顺磁特性:剧烈泵出效应
- 石墨烯和多壁碳纳米管对 Cu/Ti3SiC2/C 纳米复合材料的微观结构和机械性能的协同作用
- 磁性聚(N-异丙基丙烯酰胺)纳米复合材料:制备方法对抗菌性能的影响
- Pd 簇在聚(N-乙烯基-2-吡咯烷酮)燃烧中的催化作用
- 研究纳米粒子聚集/附聚对聚合物纳米复合材料杨氏模量影响的两步法
- <100>拉伸载荷下钽单晶弹性特性的温度和压力依赖性:分子动力学研究
- 天然和合成纳米材料的电化学、生物医学和热特性的比较研究
- 金属和金属氧化物纳米粒子的绿色合成及其对单细胞藻类莱茵衣藻的影响
- 评估由食用菌根真菌 Tricholoma crassum 合成的蛋白质包覆金纳米粒子的抗菌、凋亡和癌细胞基因传递特性


