仿生光催化剂 Zn(II)-卟啉敏化 3D TiO2 中空纳米盒和协同增强的可见光降解的简便一锅法合成
摘要
一系列仿生光触媒锌(II) meso -四(4-羧基苯基)卟啉(ZnTCP)敏化的 3D 分层 TiO2 空心纳米盒(TiO2-HNBs)由六个具有显着 {001} 面暴露的有序纳米片组装而成(ZnTCP@TiO2-HNBs)已通过一种简便的方法成功合成-pot 溶剂热法 via 以 TiOF2 为模板的拓扑转化过程。红外光谱 (IR)、紫外-可见光谱和 X 射线光电子能谱 (XPS) 证实,ZnTCP 通过与 TiO2-HNBs 结合形成酯键,在构建 3D 中空纳米盒方面发挥了决定性作用,这也提供了转移照片激发的电子桥使 TiO2-HNB 敏感以增强可见光响应。由于ZnTCP具有优异的敏化性和仿生活性,ZnTCP/TiOF2质量比为2%(T-2p)制备的ZnTCP@TiO2-HNBs的罗丹明B(RhB)的光降解率比之前提高了3.6倍。 TiO2-HNB 在模拟日光照射(> 420 nm)下 2 小时的降解率为 99%。增强的光降解能力归因于仿生催化剂的协同可见光催化机制,不仅可以产生羟基自由基(•OH)和超氧自由基(•O2 − ) 来自 ZnTCP 敏化的 TiO2-HNBs 的激发过程,但也会产生单线态氧 ( 1 O2) 仅由仿生酶卟啉提供。此外,光催化剂在五轮后表现出良好的循环稳定性和分散性,这归因于 ZnTCP 与载体 TiO2-HNB 的强化学键合。采用电化学循环伏安法,研究了中心锌离子和母体卟啉环对仿生催化剂氧化还原性能的影响。
背景
各种有机染料在各种工业应用领域都发挥着不可替代的作用,包括纺织品、皮革或纸张的染色 [1]。然而,它们的毒性、多样性和持久性直接影响生态系统的健康,并通过受污染的饮用水供应对人类构成直接威胁 [2]。如何找到一种简单的方法去除工业废水中的合成染料污染物被认为是一个紧迫的挑战。
已经开发了多种方法来处理从废水中去除有机染料,包括物理、生物和化学方法 [3,4,5]。物理方法如吸附、混凝、离子交换和膜过滤去除有机污染物[6, 7],而物理方法只是将有机分子从水相中转移出去,并不能从根本上解决问题。此外,生物过程通常在相对温和的条件下进行,由于其固有的耗时程序和对有机污染物的不耐受性,因此难以大规模工业应用。由于利用太阳能分解染料污染物,所形成的 AuAgCuBi2O4 复合材料在模拟阳光照射下对 RhB 的降解表现出优异的光催化活性。并且复合材料表现出显着增强的光催化活性 [8,9,10,11]。基于上述考虑,有效去除废水中的有机污染物还远远不能令人满意,仍然迫切需要制定具有成本效益和环境友好的策略。基于产生活性物质(如羟基自由基)的高级氧化工艺 (AOP) 可以解决水性体系中染料破坏的问题,已被用于对多种有机污染物进行非选择性氧化 [12] .一些典型的 AOPs 技术已成功开发用于处理有机污染物,包括芬顿方案、臭氧系统、半导体光催化剂和电化学催化。近年来,集酶识别和化学催化优点于一身的仿生催化降解有机污染物因其操作简单和环境相容性好而受到广泛关注[13]。
与化学催化相比,高选择性是仿生催化的最大优势之一,它可以在温和、环保的条件下运行,可以显着减少能源投入和废物产生。近来,金属卟啉及其衍生物能够模拟过氧化物酶或含铁加氧酶的氧化特征过程,由于其良好的化学稳定性和在太阳光下的高吸收系数,在持久性有机污染物的氧化降解方面备受关注。光谱。 Zhao 报道,在离子交换树脂上负载的铁 (II) 联吡啶可以有效地激活 O2,在可见光照射下降解水中的有机染料 [14]。柯林斯合成了一系列以铁为中心的四酰胺大环配体配合物,它们能够激活 H2O2 和 O2 以降解异质水性系统中的有机污染物,从而以酶促速率模拟过氧化物酶样过程。我们的团队报告说,阴离子交换树脂 (amberlite CG-400) 上负载的四 (1, 4-二噻英) 四氮杂卟啉表现出优异的有机染料氧化降解。综合报道,发现这些金属卟啉及其衍生物均以中心铁离子为基础,因为铁离子的价态是可变的。然而,因为 Fe 2+ 更难螯合到卟啉环和大气不稳定,这个过程通常在苛刻的条件和较长的反应时间下进行。
众所周知,锌卟啉及其金属卟啉具有稳定性高、光敏性好、无毒、合成简便等吸引人的特性,受到越来越多科学家的青睐。特别是锌卟啉的光敏性可以诱导单线态氧量子产率,以用于光动力治疗和有机污染物的光催化氧化降解[15]。这些优异的性能激励我们寻找更好的方法设计易于合成的锌卟啉复合材料,以研究其对有机污染物的光催化氧化。同时考虑到锌卟啉的亲水性,在其外围的大环上引入了四个羧基。
另一个亟待解决的锌卟啉复合材料的支撑问题。与我们小组之前的报道相对应,金属卟啉和金属卟啉只有在负载在阴离子交换树脂(Amberlite CG-400)或离子交换树脂上时才表现出优异的多相光催化活性。然而,各种树脂都不能在可见光照射下产生电子和空穴对并快速转移电荷。因此,我们将目光聚焦在典型的半导体光催化剂TiO2上。
迄今为止,锐钛矿型TiO2由于其无毒、较高的化学稳定性和光稳定性,在解决环境污染和能源危机(如光催化降解废水中的有机污染物)方面的潜在应用,已成为最重要的光催化材料之一。然而,由于锐钛矿型 TiO2 的带隙较宽(3.2 eV),它只能吸收太阳光中非常小的紫外线部分(约 4.5%),严重限制了其实际应用 [16,17,18]。因此,充分利用太阳光谱的丰富量来提高TiO2基光催化剂的可见光光反应活性是一个非常重要的问题,但仍然是一个很大的挑战。迄今为止,已有大量研究致力于通过半导体耦合 [19,20,21]、金属和非金属离子掺杂 [22, 23] 和表面敏化改性来提高 TiO2 的可见光利用率[24,25,26]。这可以通过以下事实来解释:表面氧空位充当光诱导电荷受体,吸附位点抑制光生电荷的复合,导致光催化反应中光生电子和空穴的可用性增加[27,28,29]。在半导体耦合方面,虽然它可以有效提高光生电子-空穴对的分离率,但它们的光反应效率强烈依赖于它们的结结构,只能获得太低的能量转换效率,无法用于工业应用[30,31,32]。 TiO2 的掺杂改性通常很难通过高温晶格交换和多步程序制备。表面敏化方法可以显着扩展 TiO2 的光响应范围,从紫外到可见光范围。作为一种良好的敏化剂,金属卟啉及其衍生物敏化的 TiO2 因其在太阳光谱内的吸收系数高、单三线态分裂小、系统间交叉量子产率高、三线态寿命长和良好的化学稳定性 [33, 34]。更重要的是,卟啉敏化能够将 TiO2 光吸收的带隙扩展到可见光区域,可以有效地催化有机污染物的降解。卟啉环周围的官能团是染料激发态电子转移到TiO2导带的通道[35, 36]。
最近,通过各种方法合成了大量的 3D TiO2 中空纳米结构,例如由纳米线、纳米片、纳米棒或纳米粒子构成的 3D 中空纳米管或微球。然而,这些由随机组装的积木组成的中空结构主要是基于类球形结构。追求由有序分层结构组成的非球形 3D TiO2 中空纳米材料,例如盒状或立方体状结构,仍然是一个挑战问题。迄今为止,实验和理论分析都表明锐钛矿 TiO2 的高能 {001} 面可以有效地分离光生电子 - 空穴对并缩小 TiO2 的带隙,这引起了研究兴趣的爆发[37]。基于以上对形态和面修饰的考虑,如何构建由六个有序排列的薄片组装而成的 3D 分层 TiO2 空心盒状结构,主要暴露 {001} 面以增强可见光吸收仍然是一个难题.因此,迫切需要开发一种简便的一锅拓扑合成策略来合成卟啉敏化的 3D 空心 TiO2 结构以获得可见光驱动的响应。据我们所知,由原位氟化物诱导自转化立方TiOF2空心纳米盒通过溶剂热处理制备锌卟啉敏化TiO2复合光催化剂尚未见报道。
在这里,我们提出了 meso -四(4-羧基苯基)卟啉锌(II)(ZnTCP)在卟啉外围具有羧基作为敏化剂,并采用简便的一锅拓扑溶剂热法制备了ZnTCP敏化的TiO2中空纳米盒(ZnTCP@TiO2-HNBs)通过在 TiO2-HNB 和锌卟啉之间形成稳定的共价键,显着的 {001} 面暴露。我们系统地研究了锌卟啉质量含量对锌卟啉修饰的TiO2中空纳米盒(ZnTCP@TiO2-HNBs)结构和可见光催化活性的影响。
方法
材料
所有试剂和溶剂均为试剂级质量,购自商业供应商(武汉国耀化学试剂有限公司),未经进一步纯化直接使用。所有溶液均用蒸馏水配制。
ZnTCP 敏化 TiO2-HNBs (ZnTCP@TiO2-HNBs) 的合成
首先制备TiOF2前体作为纳米立方体模板
在典型的程序中,将 30 mL 醋酸盐、5 mL 氢氟酸和 15 mL 钛酸四丁酯逐滴加入 100 mL 聚氟乙烯烧杯中,并连续搅拌 25 分钟。然后,将混合物转移到干燥的 100 毫升聚四氟乙烯内衬高压釜中,并在 200°C 下保持 12 小时。冷却至室温后,白色粉末用乙醇洗涤数次,60℃真空干燥过夜,即得TiOF2纳米立方前驱体。
锌卟啉 (ZnTCP) 的制备
制备过程包括两个步骤。第一步是卟啉 (TCP) 的合成:通常将 100 mL 丙酸和 4.341 g (28.8 mmol) 4-羧基苯甲醛加入 500 mL 三颈烧瓶中,在油浴中加热并搅拌 30 min以确保完全分散。将 10-mL 丙酸和 2-mL 吡咯的溶液缓慢滴加到上述系统中,并在 140°C 下回流 2 小时。将混合物冷却至室温并用150-mL热水洗涤两次。然后用碳酸钠和硫酸分别去除焦油和调节pH值在5-7之间。产物用丁醇萃取3次;最后,将产物通过旋转蒸发浓缩和干燥。 1 TCP 的 H NMR 谱显示了预期的信号,例如 -OH、Ar-H(苯基)和 -CH(吡咯)质子分别位于 4.12、6.98-7.02 和 7.46-7.57 ppm。由于平面结构的 18 个 π 电子系统,内核 NH 质子在 =− 3.34 ppm 处表现为氘可交换的广泛化学位移。 IR (KBr, cm −1 ) 光谱,-OH 为 3363,C-H 为 2927、2852,C-H 为 1712 (C=O),C=C 为 1623、1488 和 1376,COOH 为 1053,证实了 TCP 结构。质谱在m/z处显示出一个分子离子峰 =614.14 [M + 1] + . UV-vis [DMF, max/nm] B 波段在 419 nm,Q 波段在 553 和 598 nm。
其次,ZnTCP的合成如下:将0.544g卟啉和0.20g乙酸锌溶解在DMF溶剂中。使用油浴在连续搅拌下将混合物溶液在 150°C 下加热和回流 2 小时。冷却至室温,将产物浓缩,待紫色完全沉淀后加入去离子水100mL。最后,通过旋转蒸发和 meso 除去水 得到-四(4-羧基苯基)卟啉锌(II)(ZnTCP)。在 1 ZnTCP的H NMR谱,内核NH质子消失;其他质子在 4.12、6.98-7.02 和 7.46-7.57 ppm 处显示出类似的化学位移。从红外光谱来看,C=O 键振动的尖峰在 1720 cm -1 环四聚反应后增强,3352 cm -1 对于 -OH, 2928, 2853 cm −1 对于 C-H, 1619, 1504 cm −1 和 1378 cm − 1 对于 C=C,1231 和 1017 cm −1 对于 C=O,证实了 ZnTCP 的结构。 ZnTCP 的质谱数据在 m/z =678.11 [M + 1] + 处包含一个强峰 为母环离子。 XPS 光谱证明存在结合能为 1021.5 eV 的 2p1/2 Zn(II) 峰和结合能为 1045.6 eV 的 2p3/2。 UV-vis [DMF, max/nm] B 波段在 432 nm 和 Q 波段在 561 和 600 nm。 ZnTCP的合成过程如Scheme 1所示。
<图片>
ZnTCP配合物合成示意图
一种简便的一锅法合成的 ZnTCP 敏化 TiO2-HNBs (ZnTCP@TiO2-HNBs)
以TiOF2为模板,乙醇为溶剂,锌卟啉为敏化剂,TiO2中空纳米盒为载体,通过拓扑转化过程制备出具有暴露高能{001}面的3D分层二氧化钛中空纳米盒。在典型的程序中,将 300 mg TiOF2、70 mL 乙醇和 3 mg ZnTCP 放入衬有聚四氟乙烯的高压釜中,在 200 °C 下反应 48 小时,然后将其冷却至室温;产物通过旋转蒸发浓缩并在 60°C 下真空干燥过夜。所得产物记为T-1p,其中1代表ZnTCP/TiOF2质量比。为了比较,除了锌卟啉的量外,还在其他相同条件下合成了不同样品。它们分别表示为 T-0p、T-1p、T-2p、T-3p 和 T-5p。 ZnTCP@TiO2-HNBs的一锅法合成流程如图2所示。
<图片>
ZnTCP@TiO2-HNBs制备示意图
特征化
傅里叶变换红外光谱 (FT-IR) 在 NeXUS 470 光谱仪上使用 KBr 颗粒技术测量。这些催化剂的晶体结构通过粉末X射线衍射(XRD)表征,扫描速率为0.05°s -1 在 2θ 范围从 10 到 80°,在使用单色化 Cu-Ka 辐射的 Bruker D8 Advance 中。 X 射线光电子能谱 (XPS) 在 VG Multilab 2000 光电子能谱仪上使用单色 Mg Ka 记录 2 × 10 -6 真空下的辐射 Pa。所有结合能均以表面不定碳的 284.8 eV 处的 C1s 峰为参考。通过场发射扫描电子显微镜(SEM)(日立,日本)和透射电子显微镜(TEM)(Tecnai G 2 )分析制备的样品的形貌和微观结构 20,美国)。使用 Shimadzu UV-2600 分光光度计从 240 到 800 nm 使用 BaSO4 作为背景收集 UV-vis 漫反射光谱 (DRS)。在 Hitachi F-7000 荧光分光光度计上获得光致发光 (PL) 光谱。循环伏安(CV)测量在电化学工作站上进行,电化学工作站由外部 PC 控制,并在室温下使用三电极配置。
光催化测量
所有光反应实验均在自制的光催化反应器系统中进行,所制备样品的光催化活性通过有机染料RhB(1 × 10 -5 mol/L) 作为可见光照射下的目标污染物。具体过程如下:50mg光触媒和50mL初始浓度5 × 10 -4 用 14 cm 2 超声分散将 mol/L RhB 溶液加入圆柱形反应容器中 平边和 7 cm 高,然后将混合溶液在黑暗中振荡过夜。达到吸附平衡后,通过用带有滤光片 (λ) 的 210 W 氙灯照射系统引发光催化反应> 420 nm) 以确保仅由可见光照射,系统连续用水冷却,用于将系统保持在室温。在给定的时间间隔内,收集 3.5 mL 等分试样并离心,然后除去催化剂颗粒进行分析。不同时间间隔的 RhB 浓度通过紫外-可见光谱监测。所有测量均在室温下进行。
光致发光 (PL) 技术用于研究催化剂降解过程中产生的活性物质。据珍贵报道[38],香豆素、4-氯-7-硝基苯-2-氧杂-1、3-二唑(NBD-Cl)和1,3-二苯基异苯并呋喃(DPBF)作为荧光探针检测羟基自由基(•OH),超氧自由基(•O2 − ) 和单线态氧 ( 1 O2),分别。 ZnTCP@TiO2-HNBs催化剂与水反应生成活性物质羟基自由基(•OH),然后被香豆素快速捕获,生成具有强荧光性质的7-羟基香豆素。通常,检测程序如下:在磁力搅拌下混合含有香豆素 (0.1 mmol/L) 的催化剂 (1.0 g/L) 悬浮液,然后振摇过夜。混合物用 210 瓦氙灯照射并以设定的 2 分钟间隔(或 4 分钟或 15 秒)取样。用荧光分光光度计通过相应波长激发分析滤液。
结果与讨论
TEM 和 SEM 图像
通过图 1 所示的 TEM 观察 TiOF2、TiO2-HNB 和 ZnTCP@TiO2-HNB 样品的形态和晶面。图 1a 显示 TiOF2 显示出均匀的固体立方体形状和平均尺寸为 250 nm 的光滑表面。如图 1b 所示,可以看出 TiO2-HNBs 的形态转变为均匀的空心盒状,由六个平均边长为 260 nm 的有序面组成,这与立方 TiOF2 模板一致。从图 1c-f 中仔细观察,可以发现 ZnTCP@TiO2-HNB 的 3D 中空结构在氟化物诱导的自转变立方 TiOF2 到 ZnTCP 敏化的 TiO2 中空纳米盒过程中仍显示明确的盒形形态,它们是形状良好的,一些毛茸茸的 ZnTCP 触手覆盖在空心盒的表面 [39]。获得 HRTEM 图像(参见图 1g 的插图)以确认晶格条纹约为 0.235 nm,这与锐钛矿 TiO2 的高能量 {001} 微晶面非常吻合。 ZnTCP@TiO2-HNBs晶格图的表面展示了由六个有序的显性暴露{001}面纳米片组成的3D分层空心纳米盒。
<图片>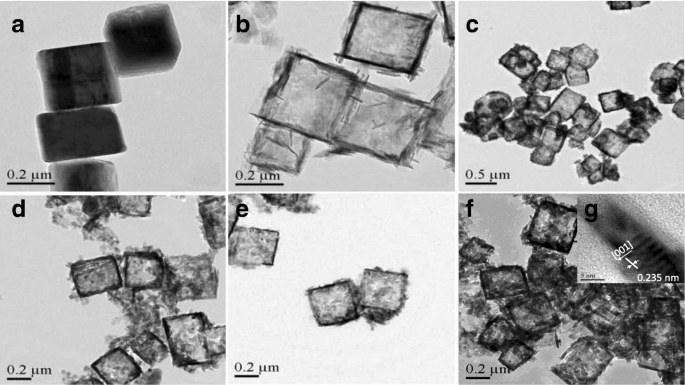
合成催化剂的 TEM 图像:a 二氧化钛,b TiO2-HNBs,c T-1p,d T-2p,e T-3p 和 f , g T-5p
TiOF2、TiO2-HNB 和 ZnTCP@TiO2-HNB 的表面形态从图 2 所示的 SEM 图像中确定。图 2a 由与 TEM 图像一致的均匀且完全立方的 TiOF2 组成。如图 2b 所示,TiO2-HNB 的图像显示出形状良好的空心盒形态,其源自拓扑转换过程中的模板 TiOF2 立方体,并被六个有序的 TiO2 纳米片阵列包围。如图 2c-f 所示,所有 ZnTCP@TiO2-HNBs 样品也表现出完全中空的盒状结构,纳米盒表面覆盖着 Zn(II) 卟啉的毛状触角,这与 TEM 结果非常一致,这意味着ZnTCP原位参与成功的从立方TiOF2到TiO2空心纳米盒的一锅拓扑转化过程。
<图片>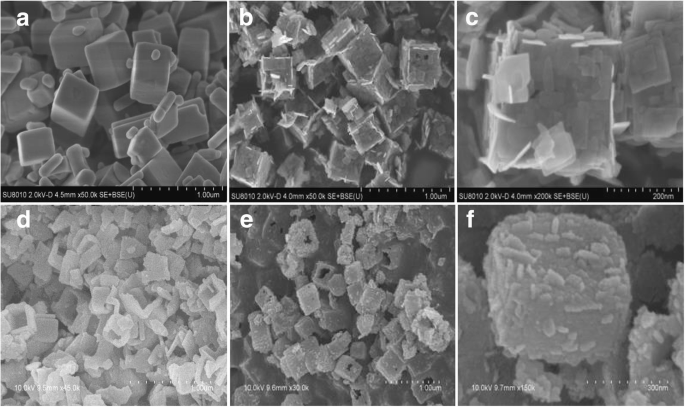
所制备催化剂的SEM图像:a 二氧化钛,b TiO2-HNBs,c T-1p,d T-2p,e T-3p 和 f T-5p
如图 3 所示,通过涉及模板参与的拓扑转换过程提出了 Zn(II)-卟啉敏化 3D TiO2 中空纳米盒 (ZnTCP@TiO2-HNBs) 的可能形成机制。乙醇通过使用溶剂提供温和的反应条件,其脱水反应(方程式 1,主要)和 ZnTCP 与乙醇之间的酯反应(方程式 2)提供了 H2O 并促进了立方 TiOF2 的水解,可拓扑转化为锐钛矿 TiO2 空心溶剂热反应过程中的盒状纳米晶体(方程 3)。由于 F − 的吸附 TiO2 纳米晶表面的离子可以显着降低 (001) 面的表面能,氟离子促进高能锐钛矿 TiO2 纳米片的形成,可以理解前驱体 TiOF2 可以通过六个有序的纳米片转化为 TiO2 中空纳米盒组装体。暴露的高能 (001) 面。 ZnTCP 的外围四羧基与载体 TiO2-HNBs 形成强酯键结合(方程式 4)。
$$ {\displaystyle \begin{array}{l}{\mathrm{TiO}\mathrm{F}}_2+2{\mathrm{CH}}_3{\mathrm{CH}}_2\mathrm{O}\ mathrm{H}\circledR {\mathrm{TiO}}_2+2\mathrm{HF}+2\mathrm{CH}={\mathrm{CH}}_2\left({\mathrm{CH}}_3{\ mathrm{CH}}_2{\mathrm{OCH}}_2{\mathrm{CH}}_3\right)\\ {}\mathrm{酒精}\;\overset{\mathrm{脱水}}{\to}\;\mathrm{烯烃}\ \left(\mathrm{ester}\right)+{\mathrm{H}}_2\mathrm{O}\end{array}} $$ (1) $$ \mathrm{Zn} \left(\mathrm{II}\right)\hbox{-} \mathrm{卟啉}\hbox{-} \mathrm{COOH}+{\mathrm{CH}}_3{\mathrm{CH}}_2\mathrm {O}\mathrm{H}\circledR \mathrm{Zn}\left(\mathrm{II}\right)\hbox{-} \mathrm{卟啉}\hbox{-} {\mathrm{COOCH}}_2{ \mathrm{CH}}_3+{\mathrm{H}}_2\mathrm{O} $$ (2) $$ {\mathrm{TiO}\mathrm{F}}_2+{\mathrm{H}}_2\mathrm {O}={\mathrm{TiO}}_2\left(\mathrm{anatase}\ \mathrm{crystal}\right)+2\mathrm{HF}\left(\mathrm{in}\ \mathrm{situtransformation} \right) $$ (3) $$ \mathrm{Zn}\left(\mathrm{II}\right)\hbox{-} \mathrm{卟啉}\hbox{-} \mathrm{CO}\mathrm{OH }+{\mathrm{TiO}}_2\hbox{- } \mathrm{O}\mathrm{H}=\mathrm{Zn}\left(\mathrm{II}\right)\hbox{-} \mathrm{卟啉}\hbox{-} \mathrm{CO}\hbox {-} \mathrm{O}\hbox{-} {\mathrm{TiO}}_2 $$ (4)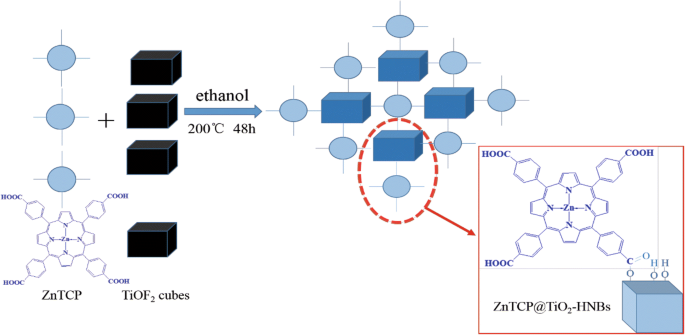
Zn(II)卟啉敏化TiO2-HNBs(ZnTCP@TiO2-HNBs)的形成过程
XRD 分析
众所周知,TiO2 基催化剂的晶面对其光催化性能起着非常重要的作用。图 4 显示了所有制备好的样品的 XRD 谱分析。查看相对于 TiOF2 前驱体的曲线,TiOF2 的 {100}、{200} 和 {210} 晶面对应于 2θ =24.05°、48.34°和54.45°被清楚地观察到。对于所有 TiO2 样品,在 2θ 处有一个宽峰 观察到=25.37°,对应于锐钛矿TiO2(JCPDS No. 21-1272)的{101}面衍射,不同于24.05°,归因于TiOF2的衍射峰,表明所有样品均成功完成了拓扑转化以 TiOF2 为模板的工艺 [40]。由于 F − 离子作为形状控制剂,在合成过程中没有形成金红石 TiO2 图案。与TiO2-HNBs(T0p)相比,Zn(II)卟啉敏化的TiO2-HNBs样品在{101}和{200}晶面附近的衍射峰变得更尖锐,表明结晶度更高,这是由于Zn(II)卟啉含量的增加导致TiO2表面羟基和ZnTCP外围四羧基之间酯化学键的浓度增加。 XRD图中TiO2样品的其他特征衍射峰没有出现任何位移或峰形变化,表明ZnTCP改性和敏化没有改变TiO2-HNBs的晶体结构。
<图片>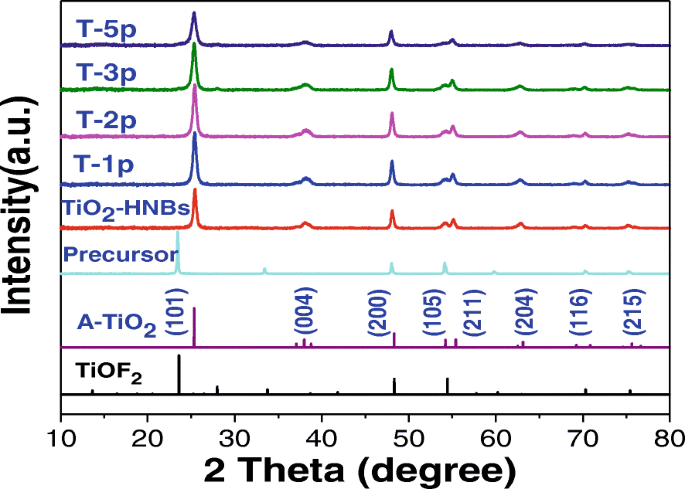
所制备样品的XRD图
为了进一步研究 ZnTCP@TiO2-HNBs 样品的微晶尺寸,基于它们的 {101} 衍射峰使用 Scherrer 方程 [41, 42]。如表 1 所述,根据结构分析,所有样品的平均粒径均在 260 nm 左右,与 SEM 和 TEM 测量结果非常一致。然而,“d ” 与 TiO2-HNBs 相比,ZnTCP@TiO2-HNBs 样品的空间值没有增加,这意味着所制备的 ZnTCP@TiO2-HNBs 催化剂的晶胞尺寸在 Zn(II) 卟啉敏化过程中不太可能发生变化。
紫外可见光谱
图5为ZnTCP在5 × 10 −4 溶液中的紫外可见光谱 mol/L DMF 溶液 (a) 和 ZnTCP@TiO2-HNBs 样品和 BaSO4 的紫外-可见漫反射光谱 (DRS) 用作参考样品 (b)。如图 5a 所示,432 nm 的强吸收峰归因于卟啉环的特征 B 带吸收,而可见光区较弱的 561 nm 和 600 nm 吸收峰归因于 ZnTCP 的特征 Q 带吸收,这意味着卟啉环合成成功。与图 5a 相比,ZnTCP 的 Q 带吸收峰在对 TiO2 敏化后红移至 660 nm,进入更长波长的可见光区。因为在水热反应后,锌卟啉与TiO2-HNBs之间呈现出很强的相互作用力,这为有机染料在可见光照射下的降解提供了有利的先决条件。可以发现,TiO2-HNBs 在其基本吸收边(> 400 nm)以上显示出较少的吸收,而 Zn(II) 卟啉敏化的 TiO2 样品显示出明显的宽可见光吸收,如图 5b 所示,并且随着 Zn(II) 卟啉量的增加,ZnTCP@TiO2-HNBs 样品的吸收强度逐渐增强。 In addition, the color of as-prepared samples become deepened gradually from white to blue purple with the increasing of mass ratio of ZnTCP/TiO2-HNBs, and it was widely accepted that the purple coloration cause Zn(II) porphyrin-sensitized samples resulted into enhanced visible-light absorption. To further investigate the bandgap in each sample, the energy gap (eV) threshold was obtained using the transversal method, and the graph of the transformed Kubelka-Munk function against the photon energy for samples T-2p and TiO2-HNBs are displayed in Fig. 5c. According to the transformed Kubelka-Munk function plot, the energy gaps of E g (T-2p) and E g (TiO2-HNBs) are 2.83 and 3.08 eV, respectively. The reduced bandgap arises because ZnTCP can form a local energy state between the valence and conduction bands as ZnTCP concentration increases, which reduce the forbidden band width and the electron transition energy. Meanwhile, we find that the ZnTCP self-doped samples also exhibit very strong UV absorption, which indicates ZnTCP self-doping not only enhances the visible-light response of the catalysts, but also improves their UV light absorption. Thus, it can be predicted that more photo-generated electrons and holes can be excited and can participate in photocatalytic reactions, which is attributed to the increased probabilities of activation by UV and visible light.
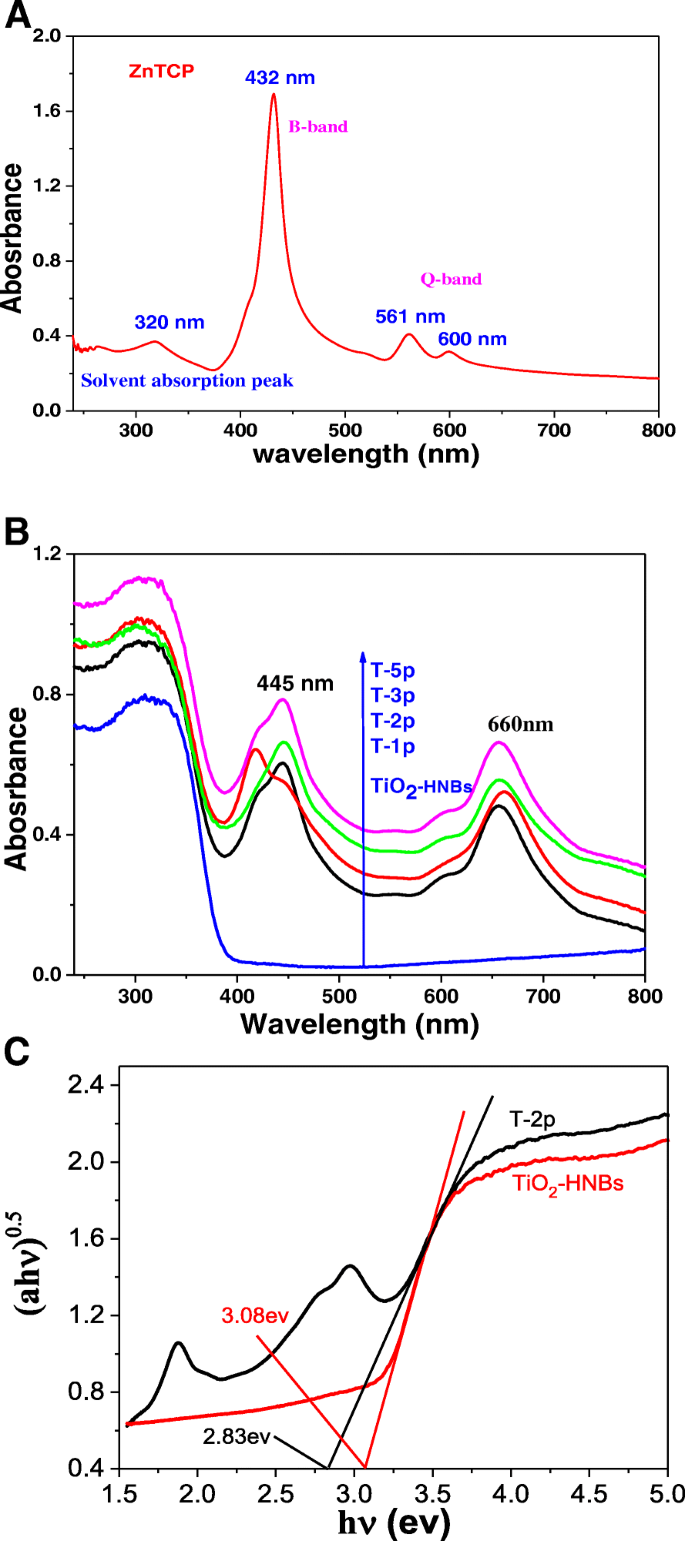
一 UV-Vis spectra of ZnTCP in 5×10 −4 mol/L DMF solution. b UV-vis diffuse reflectance spectra (DRS) of all the samples. (c ) Estimated optical absorption edges for the T-2p and TiO2-HNBs
FT-IR Spectra Analysis
FT-IR spectra analysis is a powerful tool to identify characteristic functional groups [43]. Figure 6 displays the FT-IR spectra of ZnTCP, TiO2-HNBs, and T-2p. Looking at the spectra relative to ZnTCP, the broad band at 3443 cm −1 is assigned to the stretching vibration of the -OH in the peripheral t carboxyl groups, the characteristic absorption peak of C=O at 1720 cm −1 . The stretching vibration of Ar-H bond is clearly observed at 2995 cm −1 . Furthermore, the spectra of ZnTCP show the characteristic absorption peak of benzene ring at 1620 and 1469 cm −1 relative to ZnTCP [44].
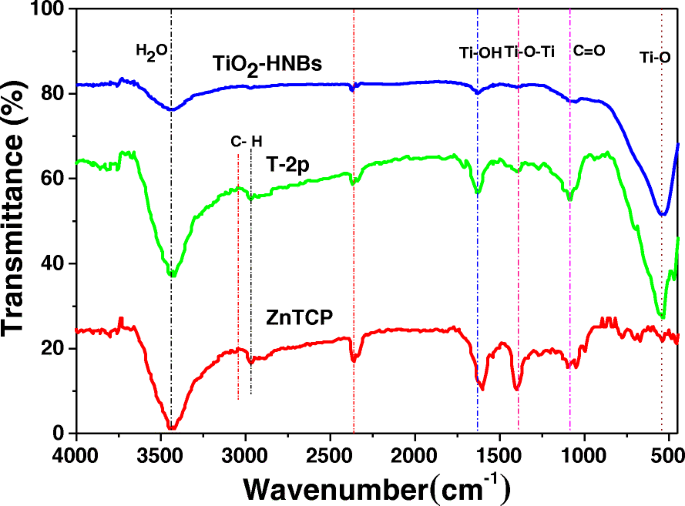
FT-IR spectra of TiO2-HNBs, ZnTCP, and T-2p sample
Seeing from the spectra relative to TiO2-HNBs, the stretching vibration and bending vibration of the absorption H2O and Ti–OH group on the surface of samples were found about 3446 cm −1 and 1627 cm −1 , 分别。 The stretching vibration of Ti-O-Ti is observed at 1388 cm −1 , and the adsorption peaks around 521 cm −1 is assigned to the bending vibrations of Ti-O bond.
Compared to ZnTCP and TiO2-HNBs, the T-2p spectra features the characteristic stretching/bending vibration of the Ar-H bond at 2996 cm −1 and 1386 cm −1 attributed to Ti-O-Ti bond. Furthermore, the spectra of T-2p shows the characteristic absorption peak of phenyl ring at 1621 and 1468 cm − 1 corresponding to ZnTCP, indicating the existence of ZnTCP on the surface of sensitized TiO2 [44]. However, the signal corresponding to -OH vibration peak disappeared, which demonstrates the formation of ester chemical bond between ZnTCP and TiO2. The stretching vibration of the C=O bond of ester group is clearly observed at 1713 cm −1 [45], further confirmed ZnTCP combined to TiO2-HNBs composites. The above information indicates that the TiO2-HNBs surface has been successfully functionalized with ZnTCP molecules. After sensitization, the spectra of T-2p show an increased intensity of the stretching vibration peak at 520 cm −1 and the characteristic absorption bands in the region of 1200~1060 cm −1 attributed to the CO-O-Ti bond resulting from the esterification between COOH of ZnTCP and OH of TiO2 [46]. This reveals that the strong interaction (conjugated chemical bonds) between ZnTCP and TiO2-HNBs was established rather than simple physical adsorption. The results displayed on FT-IR spectra were in accordance with the photocatalytic performance of these catalysts, suggesting that the strong conjugated chemical bonds between ZnTCP and TiO2 greatly contribute to the improvement of the photocatalytic activity as a transferring electrons bridge.
XPS Analysis
To further investigate chemical environments of as-prepared catalysts, the X-ray photoelectron spectra (XPS) measurements of TiO2-HNBs and T-2p were carried out [43]. As shown in full spectra of Fig. 7a, two elements including Ti and O were both observed at the corresponding positions, respectively. Figure 7b–f displayed the high-resolution XPS spectra of the corresponding element of two samples.
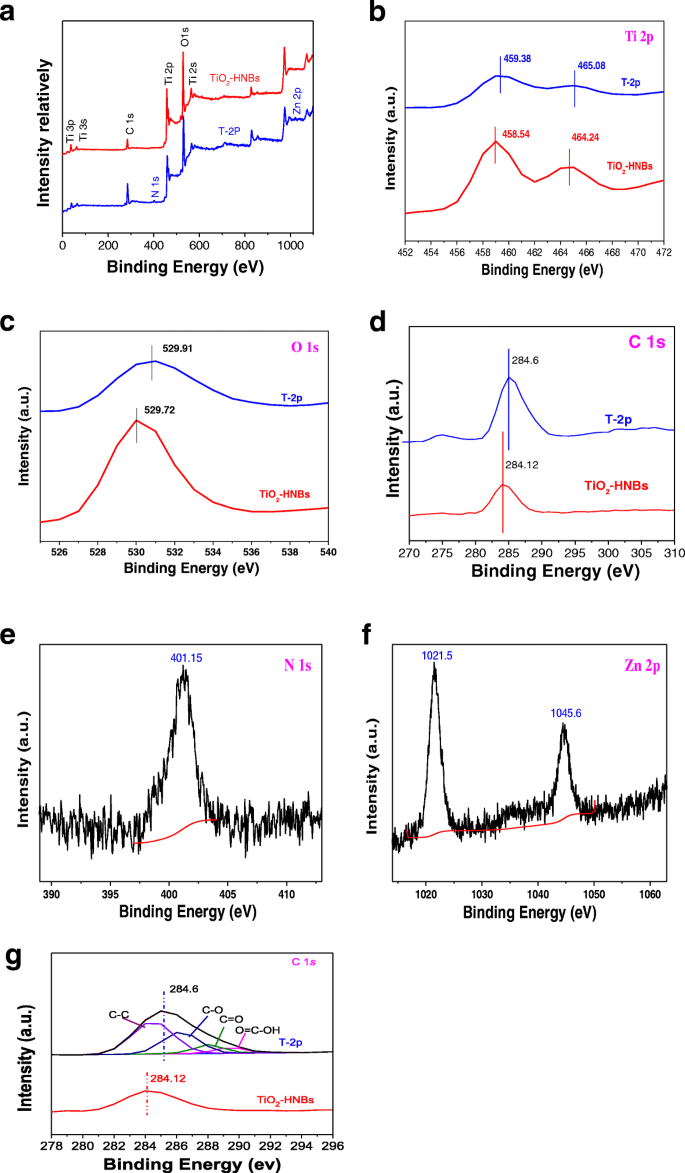
一 The full spectra of all kinds of elements in sample TiO2-HNBs and T-2p; b the high-resolution Ti 2p XPS signals of TiO2-HNBs and T-2p; c the O1s XPS signals of TiO2-HNBs and T-2p; d the C1s O1s XPS signals of TiO2-HNBs and T-2p; e the N1s XPS signals of T-2p; f the Zn 2p XPS signals of T-2p; g the C XPS-peak-differentation-imitating analysis
The high-resolution Ti 2p XPS signals of TiO2-HNBs in Fig. 7b consisted of two binding energy levels of Ti 2p1/2 and 2p3/2 at 464.24 and 458.54 eV with a separation energy of about 5.70 eV. However, the binding energy levels of Ti 2p1/2 and 2p3/2 XPS peaks of T-2p exhibited at 465.08 and 459.38 eV, which shift 0.84 eV toward the lower energy region compared to TiO2, indicative of a partial charge transferred from the surface C=O ester moiety to Ti 4+ centers, which was probably due to the strong interactions between ZnTCP molecules and TiO2.
As shown in Fig. 7c, the O1s XPS signals of TiO2-HNBs and T-2p displayed at 529.72 and 529.91 eV respectively, with a deviation energy of nearly 0.19 eV. The shifting of lattice oxygen peaks in T-2p toward lower energy, compared to TiO2 standard, further verified the different chemical environment due to ZnTCP sensitization. As displayed in Fig. 7d, the C 1s of T-2p appeared the distinct peaks at 284.6 eV, which were ascribed to C=O (and COO) bonds in the peripheral tetracarboxyl group, further confirmed that ZnTCP was well bonded on the surface of TiO2. It was found that the weak peak at 401.15 eV of T-2p in Fig. 7e was attributed to N1s of the N=N- bond in the porphyrin ring. The Zn 2p binding energy signals of T-2p appeared at 1021.5 eV and 1044.46 eV in Fig. 7f, which are basically consistent with the complex molecular structure of ZnTCP, further implying it well impregnated onto the surface of TiO2. Figure 7g shows the XPS spectra of C 1s region for samples. In Fig. 7g, the peak with a binding energy of 284.6 eV can be attributed to the C–C bonds, while the deconvoluted peaks centered at the binding energies of 286.5, 288.3, and 289.6 eV can be assigned to the C–O, C=O, and O=C–OH functional groups, respectively. C 1s spectra for the ZnTCP@TiO2-HNBs composite are shown in Fig. 7. In the spectrum of ZnTCP@TiO2-HNBs, all peaks from oxygen-containing functional groups decreased dramatically in intensity or even disappeared entirely, indicating a significant reduction of GO by solvothermal treatment. In addition, an additional shoulder peak was found, which was usually assigned to the formation of a chemical bond between a carbon atom and a titanium atom in the lattice of TiO2-HNBs, which resulted information of Ti–O–C bonds.
Photocatalytic Activity of ZnTCP@TiO2-HNBs Catalysts
The photocatalytic activity of ZnTCP@TiO2-HNBs was evaluated by measuring the degradation with RhB as a probe molecule. Figure 8a exhibits the degradation curve of RhB under simulated sun light conditions with as-prepared ZnTCP@TiO2-HNBs catalysts. It is clearly observed that, after the adsorption-desorption equilibrium process, the self-degradation of probe molecule RhB can be negligible and the visible-light degradation activities of ZnTCP-sensitized TiO2-HNBs samples indicates much higher photocatalytic efficiencies than TiO2-HNBs and ZnTCP under visible-light irradiation (λ ≥ 420 nm), which depends on the concentration of RhB versus visible-light irradiation time. The T-2p sample shows highest photocatalytic activity with RhB degradation rate of 99% after 2 h under visible-light irradiation, resulting from its enhanced visible-light response. However, the TiO2-HNBs (T-0p) shows little decomposition rate of RhB under the same reaction conditions. In this process, the order of degradation rate is T-2p> T-3p> T-1p> T-5p> ZnTCP>TiO2-HNBs.
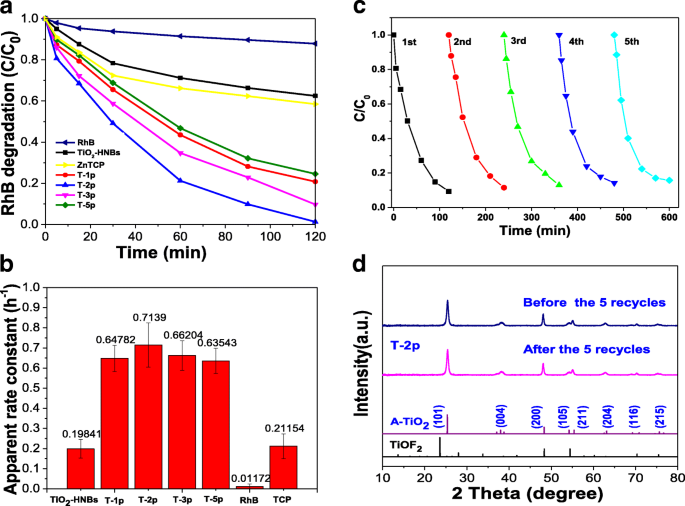
一 The C t/C0 vs. time curves of RhB photodegradation under visible-light irradiation. b The apparent first-order rate constant of RhB photodegradation. c Cyclic degradation curve of T-2p. d The XRD before and after recycles of T-2p
It is well accepted that the slopes for the curve of RhB intensity versus illumination time (rate constant) represent the photodegradation activity of the samples. Therefore, the exponential curve of the degradation RhB under visible-light irradiation was fitted in Fig. 8b, and the degradation kinetics of RhB was described by the first-order kinetics curves. Degradation experiments followed the first-order kinetic equation ln(C0-C) = − Kt + b (in which K is the apparent first-order rate constant and t is the reaction time). By comparing the rate constants (K values) of ZnTCP-sensitized TiO2-HNBs catalysts, we can see that the rate constants of these catalysts increase first and then decrease with the ZnTCP/TiO2-HNBs mass ratio increase. Observed from the rate constants histogram (Fig. 8b), we can find that T-2p reveals the highest visible-light photocatalytic activity (rate constant of 0.7139), which is 3.6 times higher than that of TiO2-HNBs (T-0p) (rate constant of 0.19841); furthermore, the photocatalytic rate constants of other ZnTCP@TiO2-HNBs samples are basically larger than that of TiO2-HNBs.
The possible reasons is that may be because ZnTCP-sensitized TiO2-HNBs can extend the absorption wavelength range of TiO2-based catalysts, reducing the bandgap of TiO2, and facilitate the effective separation of the electron-hole pairs generated by TiO2-HNBs after absorbing visible light, thereby improving the utilization of electron-hole pairs. However, too much ZnTCP-sensitized parts may result in the increase of defects on the surface of TiO2-based catalyst, resulting in a rapid recombination center of photogenerated electrons and holes, thereby decreasing the photocatalytic activity.
In order to investigate the long-term stability of as-prepared ZnTCP@TiO2-HNBs, the photocatalysts were recycled after each photocatalytic degradation experiment and then were reused in the next run after washing treatment. Figure 8c shows sample T-2p were recycled to 5 rounds after bleaching RhB under visible-light irradiation; as the number of photodegradation cycles increased, the photocatalytic rate of RhB still maintained a high catalytic activity, indicating that the active site of ZnTCP@TiO2-HNBs surface did not decrease, and the composite photocatalyst has good photocatalytic stability under visible-light irradiation. The strong bridging ester bond-linking leads to a slower decrease of the degradation efficiency for sample T-2p. These results further demonstrate that ZnTCP can establish a steady chemical bridging bond-linking interaction between ZnTCP and TiO2-HNBs, thus enhancing the visible-light photocatalytic performance, stability, and recyclability of ZnTCP@TiO2-HNBs. XRD shows that the T-2p is still anatase type and did not change the crystal type after many cycles, further verifying the stability of the catalyst (Fig. 8d).
PL Analysis
In order to study the active species generated during photocatalytic degradation, the coumarin, 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl), and 1,3-diphenylisobenzofuran (DPBF) were used as the hydroxyl radical (•OH), superoxide radical (•O2 − ), and singlet oxygen ( 1 O2) fluorescent probe molecules, respectively. Figure 9a displays the active species of hydroxyl radical detection map with T-2p as photocatalyst. As shown in Fig. 9a, the fluorescence emission wavelength was centered at 450 nm and the fluorescence intensity gradually increased, indicating the concentration of •OH group was increasing with the increase of illumination time. The reason is due to the fact that the continuously generated •OH active group and coumarin react to obtain 7-hydroxycoumarin, the amount of the reaction is gradually increased, and the fluorescence intensity is gradually increased [44, 45].
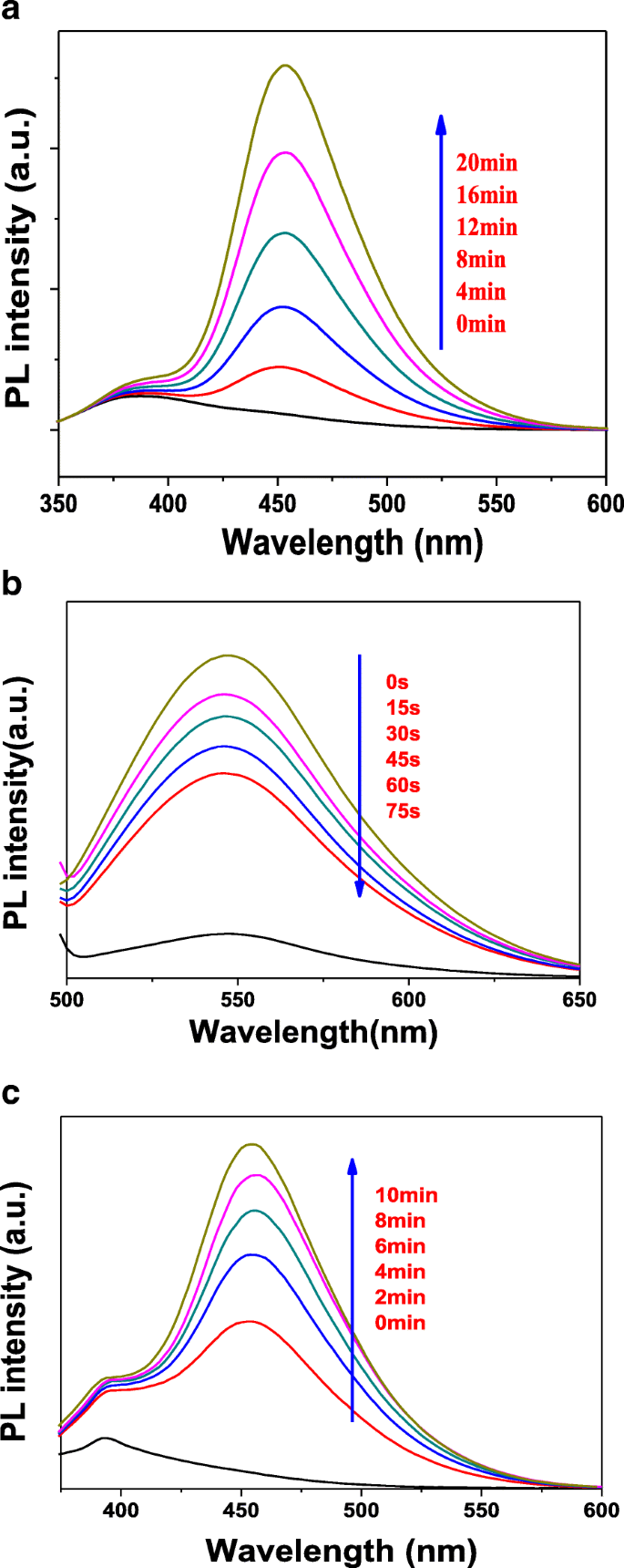
Fluorescent detection of active species •OH (a ), •O2 − (b ), and 1 O2 (c ) with T-2p as photocatalyst
It is well accepted that quantification of superoxide ion concentration can be monitored its reaction with 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) by measuring its fluorescence emission intensity at 550 nm using an excitation wavelength of 470 nm. According to Fig. 9b, it can be found that the fluorescence emission wavelength was centered 550 nm and the fluorescence intensity gradually decreased with the increase of illumination time. In the presence of NBD-Cl, the degradation rate is not obviously increased. The results indicate that •OH radical is hardly generated, while •O2 − is the significant active species generated in the system. More e − can react with O2 to form superoxide radical •O2 − instead of the rapid recombination with h + , which further improves the photodegradation activity and testifies the catalyst can generate the active species •O2 − .
In addition, a typical fluorescence method for detecting singlet oxygen ( 1 O2,) by using 1,3-diphenylisobenzofuran (DPBF) as a probe molecule is proposed, which exhibited a strong fluorescence spectrum with a maximum at 455 nm when excited at 410 nm. As shown in Fig. 9c, the fluorescence emission wavelength was centered 455 nm and the fluorescence intensity gradually increased with the increase of illumination time. Based on the above observation, it can be concluded that the photocatalyst produces a certain amount of singlet oxygen in the light condition. To sum up, the ZnTCP@TiO2-HNBs catalyst can generate •OH, •O2 − , and 1 O2 active species during the photodegradation process under visible-light irradiation.
Electrochemical Analysis
To attain more insights into photocatalytic transformation for oxidation degradation organic pollutants, the electrochemical properties of ZnTCP and ZnTCP@TiO2-HNBs (T-2p) in DMF were investigated with cyclic voltammetry (CV) on a glassy carbon electrode using tetrabutylammonium perchlorate as supporting electrolyte. It can be clearly seen that two complete oxidation and reductions processes for ZnTCP observed from the potential window are shown with blue dashed lines in Fig. 10. Furthermore, ZnTCP@TiO2-HNBs (T-2p) distinctly displayed similar two oxidation processes resulted from the ZnTCP center with red dashed lines [39]. The oxidation-reduction electrochemical behavior of ZnTCP complexes mainly occurred on the porphyrins ring and did not involve the change in the valence state of the central metal ions, which were good agreement with our previous reported [47]. However, the oxidation degradation reaction of ZnTCP@TiO2-HNBs (T-2p) was very similar as that of ZnTCP, which indicated the photocatalytic transformation mainly happened on the porphyrin ring, not relative to the center zinc ion and TiO2-HNBs. These results further confirm that the strong interaction, such as conjugated ester chemical bonds between TiO2-HNBs and ZnTCP, was established rather than simple physical adsorption interaction.
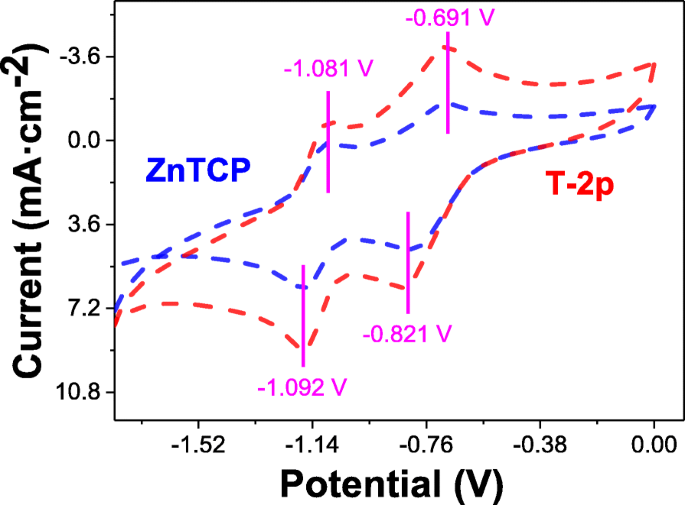
Cyclic voltammetry analysis of 1.0 × 10 −3 mol dm −3 of ZnTCP and T-2p on Pt in 0.1 mol dm −3 TBAP/DMF vs. SCE. Scan rate:0.1 V s −1
Proposed Probable Photocatalytic Mechanism
Much effort has been devoted to illustrate the photocatalytic mechanism for oxidation degradation organic pollutants. In order to speculate the photodegradation mechanism, the main oxidative species in the photocatalytic process was firstly detected through radical and hole trapping experiments by using EDTA (hole h + scavenger), p -benzoquinone (BZQ, superoxide radical •O2 − scavenger), iso -propyl alcohol (IPA, hydroxyl radical •OH scavenger) and 1,3-diphenylisobenzofuran (DPBF, singlet oxygen 1 O2 scavenger). Figure 11 shows the Ct/C0 vs. time curves of different reactive species scavengers on the photodegradation of RhB under visible-light irradiation.
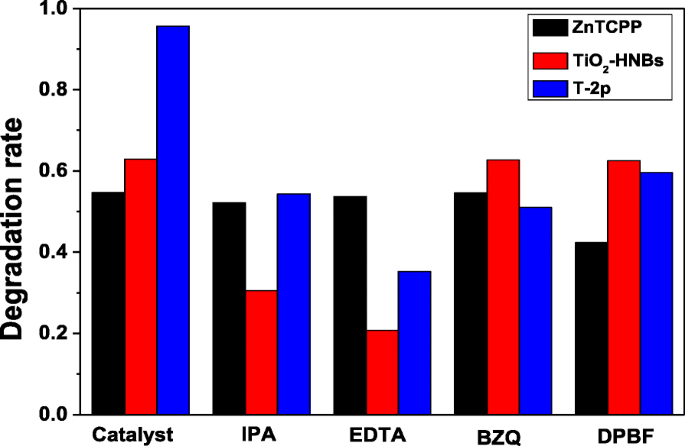
The C t/C0 vs. time curves of different reactive species scavengers on the photodegradation of RhB under visible-light irradiation
As displayed in Fig. 11, the photocatalytic degradation of RhB was apparently restrained after the injection of the active species scavenger. Taking the decreasing photodegradation rate into consideration, it can be found that RhB degradation rate with ZnTCP as a photocatalyst mainly depended on active species of 1 O2 group according to the 1,3-diphenylisobenzofuran (DPBF) detection results. Furthermore, RhB photodegradation rate with TiO2-HNBs as catalysts mostly depended on active species of h + and •OH group. However, it is very clear that RhB photodegradation rate with ZnTCP@TiO2-HNBs (T-2p) as catalysts was influenced by all active species of hole (h + ), hydroxyl radical (•OH), superoxide radical (•O2 − ), and singlet oxygen ( 1 O2), further confirming ZnTCP@TiO2-HNBs (T-2p) can generate these active species during the photodegradation process.
The probable photodegradation mechanism of organic dyes with ZnTCP-sensitized TiO2-HNBs as catalysts under visible-light irradiation is shown in Fig. 12. The degradation process generally consists of three moieties. The first part mainly involves the generation of singlet oxygen ( 1 O2). When irradiated by simulated sunlight, the inspired electrons of ZnTCP are transferred from the ground state of porphyrin [Pp] to the excited singlet state 1 [Pp]* [47], and then, relaxation of the singlet excited state generates the triplet excited state 3 [Pp]* through a process of intersystem crossing. Electrons from 1 [Pp]* and 3 [Pp]* excited states are trapped by the adsorbed O2 to form singlet oxygen ( 1 O2), which can cause RhB degradation into pieces of small organic molecule or mineralization to CO2.
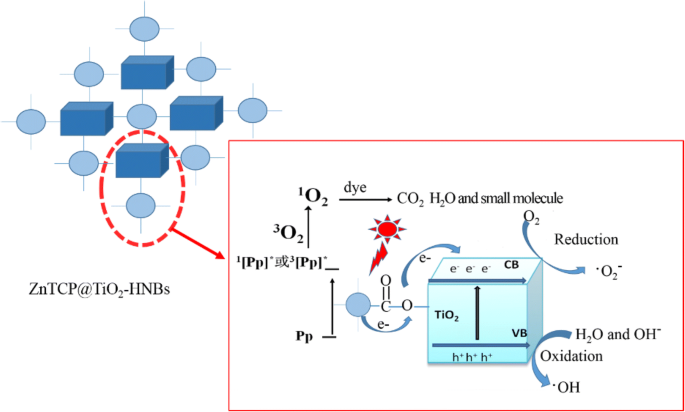
Proposed probable photocatalytic mechanism of ZnTCP@TiO2-HNBs
In contract, superoxide radical (•O2 − ) can be generated in the second moiety. Biomimetic catalytic moiety of ZnTCP is not only responsible to generate singlet oxygen ( 1 O2), but also acts as electron bridges from 1 [Pp]* and 3 [Pp]* excited states transferred to conduction band (CB) of TiO2-HNBs, which can be trapped further by the adsorbed O2, resulting in formation of an amount of •O2 − causing photodegradation of RhB present on the surface of TiO2-HNBs.
On the other hand, in the sensitization of porphyrins, the electrons of TiO2 are excited from valence band (VB) to conduction band (CB) forming electron-hole pairs (e − -h + ) during the visible-light irradiation [48, 49]. The photogenerated holes transfer to the surface of TiO2 reacted with H2O or OH − , resulting in formation of hydroxyl radical (•OH). The photogenerated electrons are transferred to the surface of TiO2 trapped by the adsorbed oxygen molecule, obtained an amount of superoxide radical (•O2 − ), and these active species (•O2 − , •OH) mainly implement the degradation of organic dyes by its successive formation of intermediaries participated.
Thus, overall, a cooperative mechanism is proposed for the degradation of organic dyes involving three components of photocatalytic system. The enhanced visible-light photodegradation activities of Zn(II)TCP@TiO2-HNBs compared to ZnTCP and TiO2-HNBs might be related to synergetic generation of three active species (•O2 − , •OH, and 1 O2), resulting in more higher efficient photocatalytic activities.
结论
A facile one-step solvothermal treatment via a topotactic transformation process was employed to synthesize a series of visible-light-driven biomimetic photocatalysts based on ZnTCP-sensitized TiO2 hollow nanoboxes assembled by six ordered nanosheets with high-energy {001} facets dominant exposure. ZnTCP played an important role in formation ester bonds to construct 3D hollow nanoboxes and transfer photogenerated electrons to sensitize TiO2-HNBs for enhancing visible-light response. Due to synergistic visible photodegradation mechanism of biomimetic catalyst, which can produce not only hydroxyl radical (•OH) and hyperoxygen radical (•O2 − ) from TiO2, but also singlet oxygen ( 1 O2) generated by biomimetic enzyme porphyrin, the photocatalytic degradation RhB rate constants of ZnTCP@TiO2-HNBs (T-2p) were greatly enhanced with a degradation yield of 99%, much larger (3.6 time) than TiO2-HNBs under visible-light irradiation. This novel approach is expected to provide a perfect reference for perfect dye-sensitized method to synergistically fabricate other surface modification TiO2-based composites, which is of great value with promising application for purifying domestic sewage.
缩写
- 3D:
-
三维
- 简历:
-
循环伏安法
- DRS:
-
UV-vis diffuse reflectance spectroscopy
- IR:
-
Infrared spectra
- PL:
-
光致发光
- SEM:
-
扫描电子显微镜
- TEM:
-
透射电子显微镜
- TiO2-HNBs:
-
TiO2 hollow nanoboxes
- UV-vis:
-
UV-vis spectroscopy
- XPS:
-
X射线光电子能谱
- XRD:
-
X射线衍射
- ZnTCP:
-
Zinc(II) meso -tetra(4-carboxyphenyl)porphyrinato
- ZnTCP@TiO2-HNBs:
-
Zinc(II) meso -tetra(4-carboxyphenyl)porphyrinato-sensitized TiO2 hollow nanoboxes
纳米材料
- 用于增强药物递送的纳米纤维和细丝
- 走向 TiO2 纳米流体——第 1 部分:制备和性质
- 小型硒纳米晶体和纳米棒的简便合成和光学特性
- Ag 修饰的 SnO2 微球的一锅绿色合成:一种用于还原 4-硝基苯酚的高效且可重复使用的催化剂
- 涂有 CuS 纳米粒子的有色导电 CuSCN 复合材料的简便合成
- 不同纵横比的银纳米线的简便合成并用作高性能柔性透明电极
- 水热合成 In2O3 纳米颗粒混合孪晶六边形圆盘 ZnO 异质结构以提高光催化活性和稳定性
- 通过蒸发诱导自组装和增强的气敏特性简便合成虫孔状介孔氧化锡
- Cu2ZnSnSe4 纳米片的一锅法合成及其可见光驱动的光催化活性
- Co3O4 纳米线的环境友好和简便合成及其与石墨烯在锂离子电池中的有前景的应用
- 石墨烯/Ag3PO4 量子点复合材料的简便一步声化学合成和光催化性能
- 掺杂 Sb2O3 纳米晶体:一种用于有机降解的高效可见光催化剂


