用银纳米粒子作为抗菌剂装饰的基于氧化石墨烯的纳米复合材料
摘要
针对耐药细菌的最有前途的方法之一是具有杀生物纳米颗粒和纳米复合材料的表面改性材料。在此,我们提出了一种在氧化石墨烯 (GO) 表面具有银纳米粒子 (Ag-NPs) 的纳米复合材料,作为一种新型多功能抗菌和抗真菌材料。超声波技术已被用作涂覆聚氨酯箔的有效方法。对革兰氏阴性菌(大肠杆菌)的毒性 ),革兰氏阳性菌(金黄色葡萄球菌 和表皮葡萄球菌 )和致病酵母(白色念珠菌 ) 通过细胞形态分析、使用 PrestoBlue 测定法评估细胞活力、使用乳酸脱氢酶测定法分析细胞膜完整性和活性氧产生来评估。与广泛用作抗菌剂的Ag-NPs和GO相比,我们的纳米复合材料对细菌和酵母细胞显示出更高的抗菌效率。
背景
抗生素的发展在控制细菌感染的数量方面发挥了重要作用。然而,抗生素的不当使用和过度使用导致许多细菌物种产生多药耐药性。一些菌株已对几乎所有常用药物产生耐药性:β-内酰胺类、四环素类和氨基糖苷类 [1]。主要耐药病原体为耐甲氧西林金黄色葡萄球菌 , 耐万古霉素肠球菌 和产超广谱β-内酰胺酶的肺炎克雷伯菌 和大肠杆菌 [2, 3]。当细菌种群的一个子集在抗生素治疗中存活下来时,具有非常大的种群和快速的增殖时间的细菌能够迅速形成抗生素抗性机制。此外,抗生素抗性细菌能够将编码抗性机制的 DNA 拷贝转移给其他远缘细菌,然后这些细菌能够将抗性基因传递给后代。因此,抗生素抗性细菌的出现代表了一个严重的问题,可以通过开发新型抗微生物剂来克服。抗菌剂在纺织工业、水消毒、医药和食品包装中非常重要。纳米粒子和纳米材料可用作抗生素的替代品 [4]。纳米粒子的抗菌活性机制因不同类型的纳米粒子而异。虽然一些提议的机制与纳米颗粒的理化结构有关,但其他机制与纳米颗粒表面抗菌离子的释放增加有关。针对微生物的多种同时作用机制需要在同一微生物细胞中发生多种同步的 DNA 突变才能产生耐药性;因此,细菌细胞很难对纳米颗粒和纳米材料产生抵抗力。抗菌纳米材料,如银、铜、富勒烯和单壁碳纳米管,由于其独特的物理化学性质和高表面积,可能具有多种优势 [5,6,7,8]。纳米颗粒 (NP) 对各种细菌的毒性的确切机制尚不完全清楚。根据目前的研究,NPs 抗菌作用的主要过程是细菌细胞膜的破坏、金属离子的释放、ROS 的产生、细菌细胞膜的渗透和细胞内抗菌作用的诱导,包括与 DNA 和蛋白质 [9, 10]。 NPs 能够通过静电相互作用附着在细菌膜上并破坏细菌膜的完整性。 NPs 表面的正电荷对于粘附是必不可少的。正电荷使纳米颗粒与带负电荷的微生物细胞膜之间产生静电加成 [11]。 NPs 与细菌细胞表面的含硫蛋白质之间的静电连接会导致细胞壁结构发生不可逆的变化,从而导致细胞壁和细胞膜的损伤 [12]。无论细胞的代谢状态如何,细菌膜都是至关重要的,因为它为细胞稳态和代谢能量转导提供了选择性渗透性。 NPs 的第二个抗菌和抗真菌活性是由于它们产生 ROS 和自由基种类的能力 [13]。 ROS水平升高引起脂质、蛋白质和DNA的过氧化[14]。
此外,许多类型的 NPs 的结构适合携带抗菌剂 [15, 16]。载体可以帮助保护药物免受目标细菌的耐药性。基于纳米颗粒的药物输送系统可以帮助将抗生素靶向感染部位,从而最大限度地减少全身副作用。其他优点包括提高疏水性药物的溶解度,延长全身循环时间和药物半衰期,以及缓释药物[4]。
最近,已经证明石墨烯是一种新的碳同素异形体,具有抗菌活性。石墨烯是一种由碳原子以六边形重复图案结合在一起的材料。石墨烯薄片的一个独特特征是其厚度与表面的比率。石墨烯的表面覆盖着电子云,这可能使这种材料易于成为电子供体,并赋予它形成特殊键的能力。石墨烯的边缘有其他键(金刚石sp3型键的特征),这些地方可能具有不同的理化特性[17]。这些特性表明石墨烯可以暴露于不同细胞间结构的塑料粘附,包括细菌细胞 [18,19,20]。此外,由于它有两个活性面(表面和边缘),石墨烯可以将生物分子附着在其边缘并粘附到细胞表面。由于氧官能团的存在,氧化形式的石墨烯,氧化石墨烯(GO)很容易分散在水和其他有机溶剂中。含氧基团可以通过共价和非共价相互作用对 GO 片进行直接的化学功能化。已经报道了GO的强抗菌活性。 GO 的抗菌活性被认为是由氧化石墨烯纳米片的尖锐边缘引起的膜应力引起的,这可能导致细胞膜的物理损伤,导致细菌膜完整性的丧失 [21]。最近,石墨烯功能化的抗菌纳米粒子已被用作有前途的抗菌材料 [22, 23]。纳米复合材料可以克服单个组件的局限性。例如,附着在石墨烯基材上的抗菌纳米材料更稳定且分散性更好 [24]。这些纳米复合材料可能含有金属、金属氧化物和聚合物。
针对耐药细菌的最有前途的方法之一是具有杀生物纳米粒子的表面改性材料。超声波技术已被证实是一种用抗菌和杀菌物质涂覆各种材料的有效方法 [25,26,27,28]。许多研究人员将超声方法归类为“绿色技术”[29, 30]。该方法基于空化现象的使用,即空化气泡在液体介质中的形成、生长和破裂 [31, 32]。内爆气泡在短时间内在高达 5000 K 的微区域中产生大量能量,并在短时间内产生高达 2000 atm 的压力 [33, 34]。因此,产生了冲击波和所谓的指向固体表面的微射流 [35]。 NPs 位于液体介质中,受到内爆效应和高速 (> 100 m/s) 喷射流的驱使,在固体表面形成一层 [36]。声空化还可以导致经超声处理的物体的物理特性发生变化,例如,调整 GO 薄片的大小 [37, 38]。
我们在之前对肠道沙门氏菌的研究中取得了可喜的成果 和单核细胞增生李斯特菌 用原始石墨烯、GO 和还原的 GO [20] 处理。在不同类型的石墨烯中,还发现 GO 在低浓度下具有最高的抗菌活性。细菌细胞分布在 GO 的整个表面。在这项研究中,我们假设用银纳米粒子 (GO-Ag) 装饰的 GO 对微生物细胞的毒性影响比裸 GO 或银纳米粒子 (Ag-NPs) 更强。因为它有两个活性面(表面和边缘),GO 氧化物可以将 Ag-NPs 连接到边缘并粘附到电池表面。石墨烯基纳米复合材料的抗菌活性可能是由于细胞膜的破坏和氧化应激。本研究的目的是评估使用革兰氏阴性菌(大肠杆菌 ),革兰氏阳性菌(金黄色葡萄球菌 和表皮葡萄球菌 )和致病酵母(白色念珠菌 ) 使用体外模型。研究包括使用 X 射线衍射、拉曼光谱透射、FT-IR、电子显微镜 (TEM)、扫描电子显微镜 (SEM) 和原子力显微镜 (AFM) 对纳米复合材料进行结构分析、微生物细胞形态评估、评估PrestoBlue™ 检测的细胞活力,乳酸脱氢酶检测 (LDH) 的细胞膜完整性研究,以及活性氧 (ROS) 产生的评估。
方法
氧化石墨烯的合成、修饰和表征
在这项研究中,市售石墨粉(Acros Organics,新泽西州,美国)通过改进的 Hummers 方法进行氧化 [39]。在 10°C 以下将 10 克石墨粉与 230 毫升浓硫酸 (98%) 混合。然后,将 4.7 克硝酸钠和 30 克高锰酸钾逐渐添加到硫酸和石墨混合物中,同时保持温度低于 10°C。然后,将混合物加热至 30°C 并搅拌 2 小时。在下一步中,加入 100 mL 水,混合物温度达到 ~ 100 °C。最后,混合物用 10mL 过氧化氢处理。为了纯化,将浆液过滤并用去离子水洗涤直至滤液的pH值达到6.5。
使用 X 射线粉末衍射仪(CuK α1 )(X’Pert PRO,帕纳科,阿尔梅洛,荷兰)。
使用由 Elementar Analysensysteme GmbH (Langenselbold, Germany) 生产的 Vario EL III 装置对 GO 中按重量计的碳、氢、氮和硫含量进行分析。在对 GO 进行化学分析测量之前,样品在解吸站(VcPrep 061,Micromeritics,Norcross,GA,USA)中在真空(0.05 毫巴)和 50°C 下进行 24 小时解吸。氧含量的计算方法是从100%的重量中减去测定的碳、氢、氮和硫的含量。
拉曼光谱使用 inVia 拉曼显微镜(雷尼绍,英国)进行。使用 514 nm 激光波长及其初始功率的 5% 分析氧化石墨烯。从样品上的五个不同点收集光谱。曝光时间为 10 秒,采集了两次扫描。
FT-IR 测量使用 Nicolet iS10 光谱仪(Thermo Fisher Scientific,美国)以衰减全反射模式在金刚石晶体上进行。将 5 微升氧化石墨烯水悬浮液滴在金刚石晶体表面并使其干燥。干燥后,在 400–4000 cm -1 范围内收集光谱 .
使用由 Malvern Instruments Ltd. (Malvern, UK) 生产的 Zetasizer Nano-ZS ZEN 3600 分别使用动态光散射 (DLS) 模式和激光多普勒电泳在室温 (23° C).
纳米材料的 TEM/SEM/AFM 分析
使用透射电子显微镜(TEM;JEM-1220 JEOL,日本东京,加速电压为 80 kV)和扫描电子显微镜(SEM;Zeiss,Ultra Plus,Oberkochen,Germany)测定粉末和箔的形态。通过将水胶体液滴置于 TEM 网格(Formvar on 3 mm 200 Mesh Cu Grids, Agar Scientific, Stansted, UK)来制备用于 TEM 观察的样品。将液滴风干后立即将网格插入显微镜中。
对于 SEM 分析,使用溅射镀膜机(SCD 005/CEA 035,BAL-TEC,Pfäffikon,瑞士)在样品上涂上薄碳层。应用了内部实验室测量程序(P5.10,2015 年 8 月 26 日第 6 版)。
使用 Asylum Research MFP3D Bio 软件(版本:Asylum Research MFP3D 15.106.09)进行 AFM(原子力显微镜)成像。使用两种成像模式对测试箔表面上的 GO 进行表面形貌成像和检测,AC 模式用于相差成像,横向力显微镜 (LFM) 用于 GO 检测,因为 GO 减少了摩擦力 [40]。
涂有 GO 和 Ag-NPs 的聚氨酯箔的制备
为了覆盖聚氨酯箔,使用了 Ag-NPs(HydroSilver1000,Amepox,Łódź,Poland)和 GO 的悬浮液。 GO、Ag-NPs 和 GO-Ag(GO (200 μg/mL)、Ag-NPs (100 μg/mL)、GO (200 μg/mL) + Ag-NPs (100 μg/mL))的悬浮液为在去离子水中制备(电导 0.09 μS/cm,去离子器:HLP 20UV,Hydrolab,Staszyn,波兰)。悬浮液无需额外纯化和过滤即可使用。
在体积为 50 毫升的玻璃烧瓶中进行聚氨酯箔的超声涂层(15 × 15 × 0.05 毫米)。将箔样品固定在支架(特氟龙)上,然后浸入制备好的悬浮液中。使用超声波喇叭(Ti 喇叭,Ø13 毫米,60% 效率,20 kHz,Sonics &Materials, Inc., Newtown, CT, USA)进行涂层过程,该喇叭与悬浮液中存在的箔样品成正方形。工艺温度为 30 ± 1°C。覆盖的样品用去离子水冲洗并在层流室中干燥,然后包装在无菌包装中。
表面自由能
润湿性测试使用 Data Physics OCA – 20 测角仪(DataPhysics Instruments GmbH,Filderstadt,德国)进行。表面自由能 (SFE) 使用 Owens、Wendt、Rabel 和 Kaelble (OWRK) 方法计算,使用两种测试液体:去离子水和二碘甲烷 [41]。
细菌和酵母的培养与制备
金黄色葡萄球菌 (ATCC 25923) 和表皮葡萄球菌 (ATCC 14990),大肠杆菌 (ATCC 25922) 和 白色念珠菌 (90028) 来自 LGC 标准 (Lomianki, 波兰)。菌株以 20% (v /v ) 甘油在 - 20 °C。在将它们用于实验之前,将菌株解冻并通过用蒸馏水洗涤细菌细胞来去除甘油。然后在以下营养培养基上培养细菌和酵母:S 的胰蛋白酶大豆琼脂 . 金黄色葡萄球菌 和 E . 大肠杆菌 , S 脑心琼脂 . 表皮 , 和用于 C 的 Sabouraud 琼脂 . 白化病 (默克密理博,德国达姆施塔特)。通过用无菌蒸馏盐水溶液轻轻洗涤平板来收获在琼脂平板上生长的细菌和酵母。为了计算细胞悬浮液中的细菌数量,使用分光光度计(Helios Epsilon,Unicam,Milwaukee,WI,USA)测量 600 nm 处的悬浮液的光密度(OD600)。通过连续十倍稀释(高达 10 − 5 ) 已知光密度的细菌和酵母悬浮液。将 1 毫升的每种稀释液涂在含有营养培养基的培养皿上。在 37°C 下孵育 24 小时后,计算培养皿上形成的菌落数。根据计数结果(一式三份),计算原菌悬液的菌落形成单位(CFU)/mL密度。
抗菌检测
用于抗菌试验的接种物是由活跃生长的生物体(对数相)制备的。所有微生物的接种物均由在 37°C 下在 Mueller-Hinton (MH) 肉汤中有氧生长的过夜培养物制备。通过测量 600 nm 处的光密度 (OD600) 确定细菌和酵母浓度。简而言之,从过夜培养物中制备细菌和酵母悬浮液并调整至 10 6 CFU/毫升。通过擦拭将接种物均匀地接种到培养皿中的MH琼脂表面。涂有 GO、Ag-NPs 和 GO-Ag 的无菌箔被沉积到琼脂表面。没有纳米颗粒的箔用作对照组。在 37°C 下孵育 24 小时后测量箔下细菌和酵母的生长情况。
可行性分析
使用 PrestoBlue™ Cell Viability Assay(Life Technologies,Taastrup,Denmark)评估细胞活力。 PrestoBlue™ 试剂会被代谢活跃的细胞迅速还原,提供活力和细胞毒性的定量测量。将细菌和酵母细胞培养在涂有 GO、Ag-NPs 和 GO-Ag 的箔上,这些箔位于插入 6 孔板(200 μL MH 肉汤,含 5 × 10 3 细胞/箔)并孵育 24 小时。在下一步中,将 90 μL 的每个样品转移到 96 孔板,并将 10 μL PrestoBlue™ 试剂添加到每个孔中,并在 37°C 下再孵育 2 小时。在酶联免疫吸附测定 (ELISA) 读数器 (Infinite M200, Tecan, Durham, NC, USA) 上在 570 nm 处记录每个孔的光密度。细胞活力表示为百分比 (ODtest − ODblank)/(ODcontrol − ODblank)×100%,其中 ODtest 是暴露于测试箔的细胞的光密度,ODcontrol 是对照样品的光密度,ODblank 是光密度无细菌和酵母细胞的孔密度。
膜完整性
LDH 测试(体外毒理学分析试剂盒,基于乳酸脱氢酶,Sigma-Aldrich,Hamburg,Germany)用于评估细胞膜完整性。由此产生的减少的 NAD (NADH + ) 用于四唑鎓染料的化学计量转化。当测定来自培养物的无细胞培养基等分试样时,LDH 活性的量可用作膜完整性的指标。如果细胞膜受损,细胞内的 LDH 分子就会释放到培养基中。细菌和酵母细胞在箔(GO、Ag-NPs 和 GO-Ag)上培养,该箔位于插入 6 孔板(200 μL MH 肉汤,含 5 × 10 3 细胞/箔)并孵育 24 小时。在没有纳米颗粒的箔上培养的细胞用作对照。此后,将样品转移到微量离心管中并以 1200 rpm 离心 5 分钟。将 100 微升上清液转移到 96 孔板中,并将 100 μL 的 LDH 测定混合物添加到每个孔中。盖上板并在室温下孵育 30 分钟。在 ELISA 读数器(Infinite M200,Tecan,Männedorf,Switzerland)上在 450 nm 处记录每个孔的光密度。 LDH 泄漏表示为百分比 {(ODtest − ODblank) − (ODcontrol − ODblank)/(ODcontrol − ODblank)}×100%,其中 ODtest 是暴露于测试箔的细胞的光密度,ODcontrol 是细胞的光密度对照样品,ODblank为无细胞孔的光密度。
微生物的SEM分析
在进行 SEM 分析之前,准备在带有 GO-Ag 和未处理细菌的箔上孵育的细菌和酵母样品。简而言之,一滴细菌和酵母培养物(10 6 CFU/ml) 在带有 GO-Ag 纳米复合材料的箔上孵育,或者将未经处理的细菌沉积在无菌盖玻片的表面上,并在 37°C 的空培养皿中孵育 24 小时。将所有样品干燥并用金覆盖。最后,使用 SEM(FEI Quanta 200,Tokyo,Japan)在 15 kV 的加速电压下对样品进行成像。
ROS 生产
使用 DCFDA,细胞活性氧物种检测试剂盒(Abcam,Cambridge,UK)评估 ROS 的产生。 DCFDA 使用细胞渗透试剂 2',7'-二氯荧光素二乙酸酯,这是一种荧光染料,可测量细胞内的羟基、过氧化氢和其他 ROS 活性。扩散到细胞中后,DCFDA 被细胞酯酶脱乙酰化为非荧光化合物,随后被 ROS 氧化为 2',7'-二氯荧光素 (DCF)。细菌和酵母细胞在箔(GO、Ag-NPs 和 GO-Ag)上培养,该箔位于插入 6 孔板(200 μL MH 肉汤,含 5 × 10 3 细胞/箔)并孵育 24 小时。在没有纳米颗粒的箔上培养的细胞用作对照。此后,将样品转移到微量离心管中并以 1200 rpm 离心 5 分钟。将一百微升上清液转移到 96 孔板中,并将 100 μL 稀释的 DCFDA 添加到每个孔中,并在 37°C 下在黑暗中再孵育 45 分钟。 DCF 的产生通过荧光光谱法测量,激发波长为 485 nm,发射波长为 535 nm,在 ELISA 读数器(Infinite M200,Tecan,达勒姆,北卡罗来纳州,美国)上进行。
结果
GO 和 Ag-NPs 的特性
化学分析表明存在氮、碳、硫、氢和氧(表 1)。
GO 样品的物相分析(图 1)显示存在来自痕量石墨、硝酸钠和可能还原形式的氧化石墨烯的杂质。
<图片>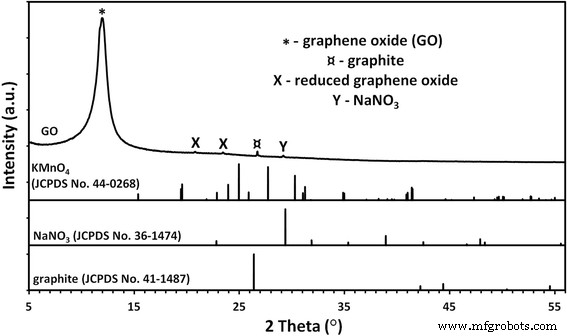
GO粉末的X射线衍射图。 GO 样品的相分析表明存在来自痕量石墨、硝酸钠和可能还原形式的氧化石墨烯的杂质
拉曼光谱可以提供有关氧化石墨烯结构特征的信息。 D带归因于结构无序,而G带来自碳sp 2 的键拉伸 原子 [42]。额外的带(包括 D'、2D 和 D + G)源于碳材料石墨结构中存在的缺陷。 我 D/我 G 比(由 D 和 G 带的强度计算)可用于表征碳材料中石墨结构的无序。从图 2 中可以看出,由于石墨粉氧化过程中形成的结构中有许多官能团,GO 具有高度无序的结构。 D 波段的位置是 1351 cm −1 和 G 波段 1590 cm −1 ; 我 D/我 G比为1.15。
<图片>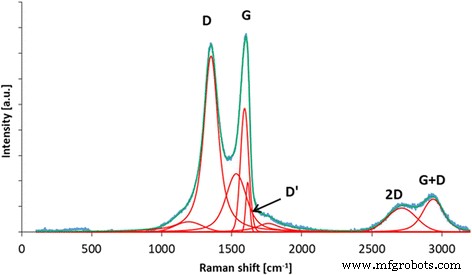
氧化石墨烯的拉曼光谱,建议对 D、G、D'、2D 和 D + G 波段进行解卷积。由于石墨粉氧化过程中形成的结构中有许多官能团,GO具有高度无序的结构。 D 波段的位置是 1351 cm −1 和 G 波段 1590 cm −1 ; 我 D/我 G比为1.15
在 ATR 模式下收集的氧化石墨烯的 FT-IR 光谱显示,GO 结构中存在大量官能团。最显着的峰值可以在 ~ 3500 cm −1 处观察到 ,主要分配给水和羟基(图 3)。 1080 cm −1 附近的一个非常密集的峰 也可以归因于羟基。 1600 cm −1 附近的峰值 通常分配给石墨碳中存在的 C=C 键。然而,我们之前的 XPS 研究表明,氧化石墨烯中存在一些 C=C 键 [43];因此,我们将此峰归因于仍然存在于氧化石墨烯中的大部分水。在 FT-IR 光谱上观察到的其他峰表明 GO 富含含有 C=O 键的基团(主要是羧基),峰位于 1720 cm -1 , 环氧树脂 (C–O–C) 可见峰在 1200 cm -1 , 和 C–H 键(峰值在 2800 cm −1 )。 FT-IR 分析与对氧化石墨烯进行的 XPS 测量非常一致,其中还确定了羟基、羧基、环氧基和羰基 [44]。比较了 10 分钟超声均化后的 GO 和 GO(图 4 和 5),类似地,比较了 10 分钟超声均化后的 Ag-NP 和 Ag-NP(图 4 和 6)。为了避免化合物形态的变化,所有化合物用液氮快速冷却并在冻干机中干燥。图 5a、b 显示了 GO 薄片,而图 5c、d 显示了超声波对 GO 薄片的影响,GO 薄片经历了部分折叠和碎裂。图 6 还显示了 Ag-NP 的类似效果,其中材料形态的变化是可见的。图 6a、b 显示了干燥的聚乙烯醇,用于稳定 Ag-NP 的水悬浮液。超声均质的破坏作用显着,因为聚乙烯醇结构被分解成具有小球形开口的长异质部分(图 6c、d)。
<图片>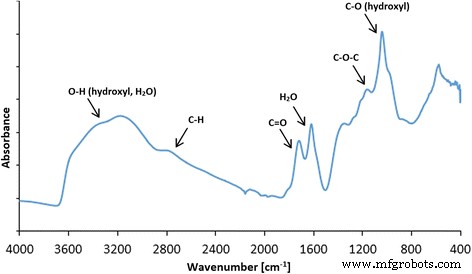
氧化石墨烯的 FT-IR(ATR,衰减全反射)光谱以及 GO 中存在的官能团的建议分配。最显着的峰出现在 ~ 3500 cm -1 ,(归因于水和羟基),~ 1080 cm −1 (羟基),~ 1600 cm −1 (分配给存在于石墨碳中的 C=C 键)。在 FT-IR 光谱上观察到的其他峰表明 GO 富含含有 C=O 的基团(主要是羧基),峰值在 1720 cm -1 , 环氧树脂 (C–O–C) 可见峰在 1200 cm -1 , 和 C–H 键(峰值在 2800 cm −1 )
<图片>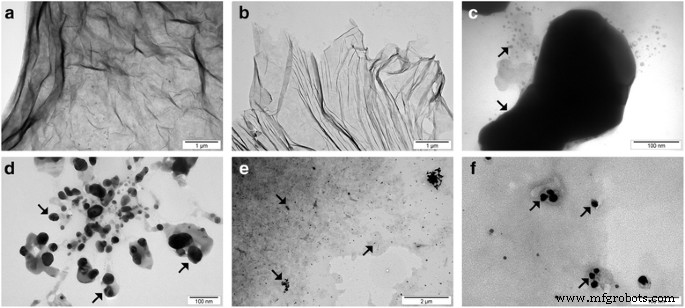
团聚的 GO 薄片 (a ), 超声波处理后的 GO 薄片 (b ), 聚集的 Ag-NPs (c ), 超声波处理后的 Ag-NPs (d ) 和 GO-Ag (e , f )。超声处理后平均 GO 粒径的减小是由 GO 薄片的碎裂或折叠引起的。超声处理后平均银尺寸的减小是由银团块的碎裂引起的。注:箭头指向 Ag-NPs/团聚体
<图片>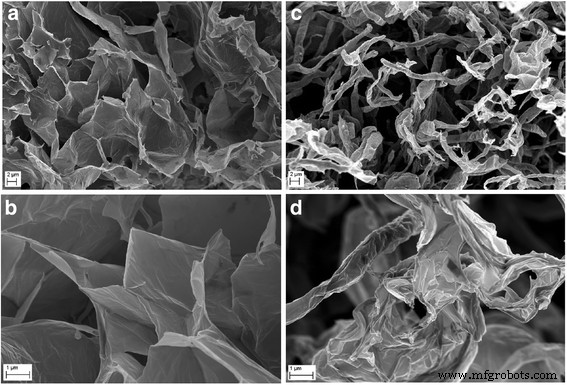
冻干 GO 薄片形貌的比较 (a , b ) 和超声波处理后的 GO 薄片 (c , d ) 使用扫描电子显微镜。超声波处理后GO平均粒径的减小是由于GO薄片的碎裂或折叠引起的
<图片>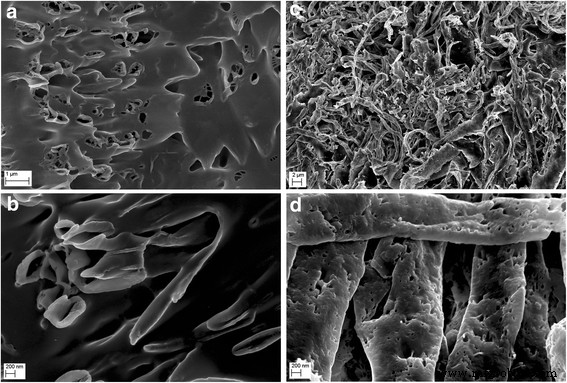
冻干 Ag-NP 混合物的形态比较 (a , b ) 和超声波处理后的 Ag-NP 混合物 (c , d ) 使用扫描电子显微镜
平均尺寸和 Zeta 电位
水悬浮液中平均颗粒/附聚物尺寸的结果列于表 2 中。对未进行超声均质化的浓缩悬浮液(原样)和稀释悬浮液进行平均尺寸分析。测试前稀释的悬浮液进行超声均质化,均质化参数与超声涂覆纳米材料层(Ag-NPs,GO)箔时使用的参数相同。对于 Ag-NP 悬浮液,超声波的作用导致平均粒径从 80 纳米增加到 218 纳米。悬浮液中超声均化后平均粒径增加的主要原因(除了 Ag-NP 团聚过程)是 Ag-NPs 被驱入用于悬浮液稳定的聚乙烯醇中。通过超声均质的 Ag-NP 样品的大标准偏差是由于松散的 Ag-NP 和 Ag-NP 的存在导致悬浮液中的聚(乙烯醇)。在 GO 悬浮液的情况下,经过超声匀浆的样品的平均粒径为 263 nm,比未经匀浆的样品的平均粒径小约 7.7 倍。所得结果与 SEM 测试(图 5)一致,显示了超声波对 GO 薄片的破坏作用。 The decrease of the average GO particle size was caused by defragmentation or folding of the GO flakes. However, it should be emphasized that the results of the average particle size of GO suspension samples involve an error related to the nanomaterial shape. The results obtained by the DLS method are a hydrodynamic average that is calculated based on the shape of a sphere that has the same diffusion coefficient as the measured particles; however, the shape of GO was flakes, which was confirmed by SEM images.
Test results of the zeta potential analysis of samples are provided in Table 3. The zeta potential of Ag-NPs in a water suspension was merely − 5.9 mV, which resulted in a lack of electrostatic stability of the sample. The sample of Ag-NP suspension was stabilized sterically by preserving Ag-NP distances through poly(vinyl alcohol) addition, which prevented agglomeration/aggregation of Ag-NPs. The zeta potential of the GO suspension sample, in turn, was − 41 mV, which gave a moderate electrostatic stability to the sample. A moderate electrostatic stability of a sample is characterized by slow sedimentation with virtually negligible change of particle size in the period of declared fitness of the suspension. The zeta potential result of the mixture of Ag-NPs and GO was − 7.1 mV, which potentially means that during the action of the ultrasounds, the GO flakes were coated by poly(vinyl alcohol) and Ag-NPs. The obtained zeta potential result of the mixture of Ag-NPs and GO sample in the water suspension implied that electrostatic stability was not present.
Foil Characteristics
In order to determine the morphology of the created layers, four types of foil samples were compared (Fig. 7):pure polyurethane foil (A, B), GO-coated polyurethane foil (C, D), Ag-NP-coated polyurethane foil (E, F), and GO-Ag mixture-coated polyurethane foil (G, H).
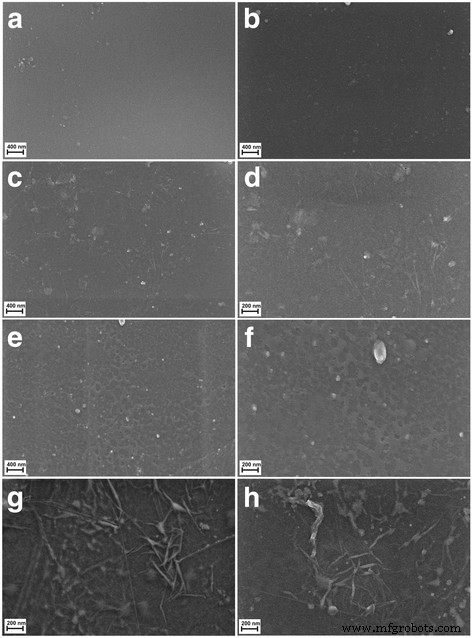
Scanning electron microscopy images of a , b non-coated polyurethane foil with a smooth surface with single impurities; c , d foil coated with GO, the broken-down GO flakes deposited on the polyurethane foil surface; e , f foil coated with Ag-NPs on which grid structures composed of polyvinyl alcohol and Ag-NPs are observed; and g , h foil coated with GO-Ag, which was mixed under the influence of ultrasounds and deposited on the foil surface
Figure 7a, b shows an uncoated polyurethane foil with a smooth surface with single impurities. In Fig. 4c, d, the broken-down GO flakes deposited on the polyurethane foil surface are noticeable. Figure 7e, f shows the foil surface coated with Ag-NPs on which grid structures composed of polyvinyl alcohol and Ag-NPs are observable. Figure 7g, h presents a mixture of GO-Ag composition, which was mixed under the influence of ultrasounds and deposited on the foil surface.
AFM Analysis and Surface Free Energy
AFM and LFM were used to complement the information about the surface morphology investigated by SEM. The investigation confirmed evolution of surface morphology by sonication of the polyvinyl alcohol with Ag-NP, GO, and GO-Ag NP solutions on the foils. Pure polyurethane foil was used as a reference foil in relation to foils coated by the ultrasonic method. The images in Fig. 8 are the AFM phase contrast images made in AC; additionally, cross sections of the GO flakes are attached under the corresponding images. Figure 8a is an image of pure polyurethane film; Fig. 7b depicts the Ag-NP-coated film, where characteristic and homogeneous grid structures are observable, being similar to those in Fig. 8e, f. Figure 8c, d shows GO-Ag-NP-coated film. Figure 8e shows the surface of the foil almost entirely covered with GO flakes; the phase contrast image helps to observe these two phases, the darker area is GO and the lighter area is polymer foil. It was noticed that the morphology of the foils has changed after Ag-NP coating comparing to the not-coated foils. The GO-Ag-NP coating differs from the previous one because it contains also small amounts of GO flakes seen as small black spots on the image, as it was mentioned earlier. Figure 8f depicts magnification of one GO flake made in LFM. The reduced friction confirms that it is, in fact, a GO flake.
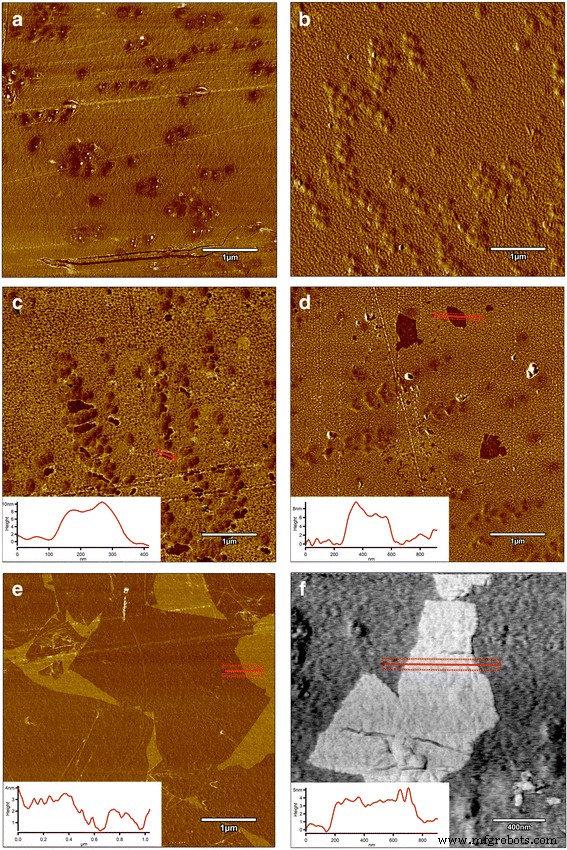
AFM phase contrast images and cross sections topographic images of graphene flakes:a non-coated foil polyurethane foil; b Ag-NPs coated foil where characteristic and homogeneous grid structures are observed; c , d GO-Ag-coated foil; e GO-coated foil, the surface of the foil almost entirely covered with GO flakes; the phase contrast image helps to observe these two phases, the darker area is GO and the lighter area is polymer foil; f LFM image of graphene flake. Red marks, area of cross section
The polar component for the GO-coated foil increased in relation to pure foil, from 2.3 ± 0.6 to 68.9 ± 2.8 mJ/m 2 , while the dispersion component decreased from 34.4 ± 1.3 to 8.2 ± 1.2 mJ/m 2 . SFE increased from 36.7 ± 1.4 to 77.0 ± 3.4 mJ/m 2 . A similar effect was not observed on foil surfaces coated with Ag-NPs and GO-Ag mixture. SFE of foil samples coated with Ag-NPs and GO-Ag mixture does not differ statistically (Table 4).
Antibacterial Properties
The antibacterial activity of the different foils coated with GO, Ag-NPs, and GO-Ag were tested with E . coli , S . 金黄色葡萄球菌 , S . epidermidis , and C . albicans. Results showed that after co-incubation with bacteria at 37 °C for 24 h, foils inhibited the growth of all tested microorganisms but to various degrees. The maximum antibacterial effect against all tested microorganisms was with foil coated with the GO-Ag nanocomposite. The bacterial growth of the cells treated with foils coated with GO and Ag-NPs was slightly lower than that of cells in the control group whereas the growth of bacterial cells treated with foils coated with GO-Ag was greatly inhibited, 88.6% of E . coli , 79.6% of S . 金黄色葡萄球菌 , 76.5% of S . epidermidis , and 77.5% of C . albicans (Figs. 9, 10, and 11).
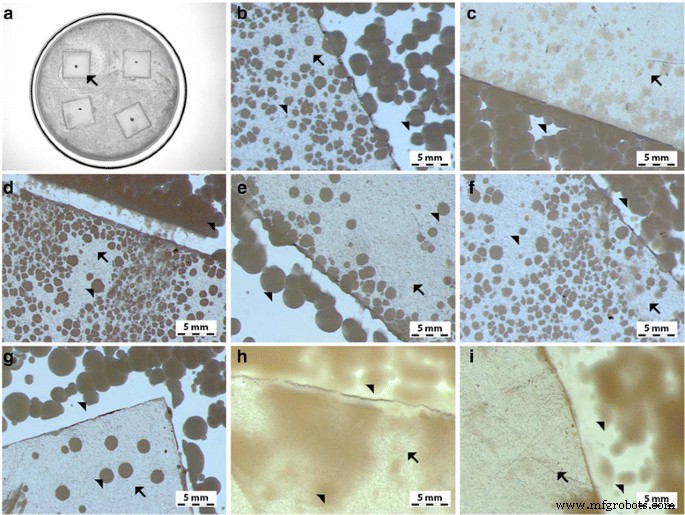
Antimicrobial properties of GO-Ag coated foils. The growth of E . coli (b , c ), S . 金黄色葡萄球菌 (c , d ), S . epidermidis (e , f ), and C . albicans (g –i ) colonies is reduced after co-incubation with GO-Ag-coated foils at 37 °C for 24 h. 一 Representative agar plate with GO-Ag-coated foils. Notes:Black arrows point to GO-Ag coated foils; arrowheads point to colonies of microorganisms
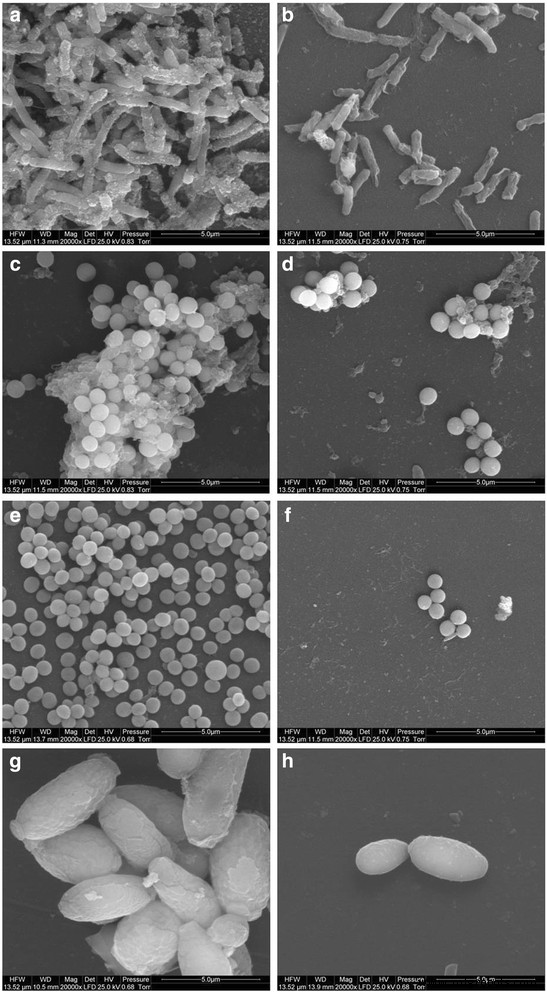
Morphology of microorganisms after co-incubation with GO-Ag-coated foils. Scanning electron microscopy images of bacteria and yeast in the control foils (a , c , e , g ) and foils coated with GO-Ag (b , d , f , h ) after incubation at 37 °C for 24 h. E . coli (a , b ), S . 金黄色葡萄球菌 (c , d ), S . epidermidis (e , f ), and C . albicans (g , h ) show decreased growth and deformed morphology after co-incubation with GO-Ag-coated foils
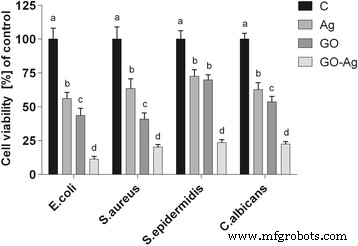
Foils coated with nanomaterials decrease E . coli , S . 金黄色葡萄球菌 , S . epidermidis , and C . albicans 可行性。 Viability of bacteria and yeast after incubation on foils coated with Ag-NPs, GO, and GO-Ag at 37 °C for 24 h was assessed with Presto Blue assay. C control group (foil without nanoparticles), Ag foil coated with silver nanoparticles, GO foil coated with graphene oxide, GO-Ag foil coated with composite of graphene oxide and silver nanoparticles. Note:The columns with different letters (a–d) indicate significant differences between the concentrations (P = 0.001); error bars are standard deviations
Membrane Integrity
In cases where the cell membrane is damaged, intracellular LDH molecules could be released into the culture medium. The LDH level outside the cells demonstrates the cell membrane integrity. Foils coated with GO, Ag-NPs, and GO-Ag disrupted cell membrane functionality and integrity with significant differences between control groups and the Ag-NPs and GO-Ag groups (Fig. 12). The highest disruption of cell membranes was observed in the GO-Ag groups, 66.3% of E . coli , 59.4% of S . 金黄色葡萄球菌 , 54.8% of S . epidermidis , and 48.5% of C . albicans .
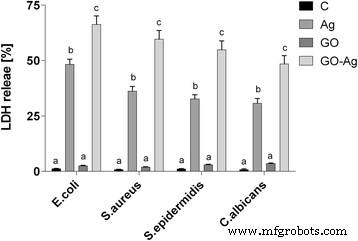
Foils coated with nanomaterials decreased E . coli , S . 金黄色葡萄球菌 , S . epidermidis , and C . albicans membrane integrity. Membrane integrity of bacteria and yeast after incubation on foils coated with Ag-NPs, GO, and GO-Ag at 37 °C for 24 h was assessed with LDH assay. C control group (foil without nanoparticles), Ag foil coated with silver nanoparticles, GO foil coated with graphene oxide, GO-Ag foil coated with composite of graphene oxide and silver nanoparticles. Note:The columns with different letters (a–c) indicate significant differences between the concentrations (P = 0.000); error bars are standard deviations
ROS Production
Low levels (or optimum levels) of ROS play an important role in signaling pathways. However, when ROS production increases and overwhelms the cellular antioxidant capacity, it can induce macromolecular damage (by reacting with DNA, proteins, and lipids) and disrupt thiol redox circuits. Foils coated with Ag-NPs and GO-Ag (P < 0.05) increased the ROS production of all tested microorganisms compared to the control group. Foils coated with GO only increased the ROS production of C . albicans . The highest ROS production was observed in the GO-Ag group (Fig. 13).
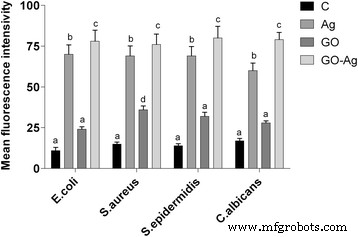
Effect of foils coated with nanomaterials on the production of reactive oxygen species. E . coli , S . 金黄色葡萄球菌 , S . epidermidis , and C . albicans were cultured on foils coated with Ag-NPs, GO, and GO-Ag at 37 °C for 24 h. Production of reactive oxygen species was assessed with DCFDA, Cellular Reactive Oxygen Species Detection Assay Kit. C control group (foil without nanoparticles), Ag foil coated with silver nanoparticles, GO foil coated with graphene oxide, GO-Ag foil coated with composite of graphene oxide and silver nanoparticles. Note:The columns with different letters (a–d) indicate significant differences between the concentrations (P = 0.000); error bars are standard deviations
Discussion
The discovery of antibiotics, natural products produced by microorganisms that are able to prevent the growth of bacteria and thus cure infectious diseases, revolutionized medical therapy; however, the overuse and misuse of antibiotics have been key factors contributing to antibiotic resistance. Now, the era of antibiotics is coming to an end, and new antibacterial agents are needed. In recent years, studies have reported nanoparticles as a promising alternative to antibacterial reagents because of their antibacterial activity in several biomedical applications [19, 45]. Nanoparticles can be an effective way to control many pathogenic and antibiotic-resistant microorganisms. Among many metal nanoparticles, Ag-NPs have been intensely studied because of the distinct properties of their antibacterial activity [7]. Ag-NPs have been proved effective against over 650 microorganisms including bacteria (both gram-positive and negative), fungi, and viruses; however, the precise mechanism of antimicrobial action is not understood completely [46]. Ag-NP exposure to microorganisms could cause adhesion of nanoparticles to the peptidoglycan and the cell membrane [47], penetration inside the cell [48], induction of ROS [49], and damaging of intracellular structures [50]. However, bare Ag-NPs aggregate when they come into contact with bacteria; thus, they lose their active surface area and show weaker antibacterial activity [51]. To overcome this problem, nanocomposites composed of graphenic materials and Ag-NPs or other metal nanoparticles could be fabricated. GO with oxygen-containing functional groups is water soluble and therefore more biocompatible than pristine graphene. As a result, Ag-based GO nanocomposites may be used as antibacterial agents. However, the information about antimicrobial properties of graphene-based composites is limited, and mechanisms of toxicity or lack of toxicity are not fully explained.
The aim of this work was to study the action of graphene oxide-based nanocomposites decorated with Ag nanoparticles on S . 金黄色葡萄球菌 , S . epidermidis , E . coli , and C . albicans 生长; membrane integrity; and ROS production. After co-incubation with the bacterial and yeast cells for 24 h, foils coated with GO-Ag nanocomposite inhibited the growth of all tested microorganisms with varying degrees, 88.6% of E . coli , 79.6% of S . 金黄色葡萄球菌 , 76.5% of S . epidermidis , and 77.5% of C . albicans . This action is most probably due to an increase in cell membrane and wall penetration by the nanoparticles. Some researchers suggest that the antimicrobial activity of graphene-based nanocomposites may be due to the disruption of cell membrane integrity and oxidative stress [52].
Foils coated with GO, Ag-NPs, and GO-Ag disrupted cell membrane functionality and integrity with significant differences between the control group and the Ag-NPs and GO-Ag groups. The highest disruption of cell membranes was observed in the GO-Ag groups, 66.3% of E . coli , 59.4% of S . 金黄色葡萄球菌 , 54.8% of S . epidermidis , and 48.5% of C . albicans . However, foils coated with bare Ag-NPs also disrupted cell membranes. It has been proposed that Ag-NPs are able to interact with bacterial membranes by increasing permeability and changing the structure of membranes, which finally leads to cell death [50]. Ag-NPs can cause direct damage to the bacterial cell membrane. Bacteria may be killed by the combined bactericidal effects of the released Ag + ions and Ag nanoparticles. Additionally, the antimicrobial potential of Ag-NPs is also influenced by the thickness of the cell wall of the microorganisms [53]. The wall of gram-positive cells contains a thick layer (20–80 nm) of peptidoglycan, which is attached to teichoic acids. In gram-negative bacteria, the cell wall comprises a thin (7–8 nm) peptidoglycan layer and contains an outer membrane. The thicker peptidoglycan layer in gram-positive bacteria, such as S . 金黄色葡萄球菌 和 S . epidermidis , may explain why these bacteria are more resistant to the antibacterial effects of GO-Ag.
Many studies have sought to establish a mechanism of action of antibacterial activity exhibited by silver in both its colloidal and ionic form. A disruption of membrane functionality from an interaction between released Ag + ions and the cell membrane and extensive cell membrane damage caused by the formation of ROS ultimately causes damage to the cell due to oxidative stress. Additionally, Ag + ions could cause dysfunction of the respiratory electron transport chain by uncoupling it from oxidative phosphorylation by inhibiting respiratory chain enzymes [54]. Foils coated with Ag-NPs and GO-Ag increased the ROS production of all tested microorganisms compared to the control group. The biological targets are DNA, RNA, proteins, and lipids. Lipids are one major target during oxidative stress. Free radicals can directly attack polyunsaturated fatty acids in bacterial and yeast membranes and activate peroxidation of lipids. A fundamental effect of lipid peroxidation is a decrease in membrane fluidity, which can significantly disrupt membrane-bound proteins. DNA is also a main target. Mechanisms of DNA damage involve abstractions and addition reactions by free radicals leading to carbon-centered sugar radicals and OH- or H-adduct radicals of heterocyclic bases. The sugar moieties producing single- and double-strand breaks in the backbone, adducts of base and sugar groups, and cross-links to other molecules can block replication. Foils coated with GO increased the ROS production at very low levels. However, Hu et al. [55] demonstrated that GO had a detrimental effect on E . coli due to decreased production of ATP and increased ROS production.赵等人。 [56] reported the antibacterial activity of GO and reduced GO. Also, Gurunathan et al. [57] presented that GO and reduced GO showed significant antibacterial activity in a concentration- and time-dependent manner. Their results demonstrated that oxidative stress is a key mechanism for the antibacterial activity of GO and reduced GO through ROS generation.南达等人。 [53] reported the effect of cystamine-conjugated GO against E . coli , S . typhimurium , E . faecalis , and B . subtilis with ROS production and high antibacterial activity.
Kurantowicz et al. [20] confirmed that bacteria could adhere to the GO surface, which results in the highest antibacterial activity. GO is characterized by a high degree of oxygenated functional groups:carbonyl, carboxylate, and hydroxyl. We hypothesize that these groups can be attractive groups for bacterial and yeast attachment. These groups are present on a large range of nutrients (amino acids, fatty acids) which are commonly recognized by microorganisms. In the present study, foils coated with GO induced membrane disruption and ROS production at a lower level than the Ag-NP and Ag-GO groups; however, cell viability was decreased, which is likely connected to the smaller active surface of GO after ultrasonic modifications.
Conclusions
Ag-NPs, GO, and Ag-GO nanocomposites demonstrated the antibacterial activity that is stronger against gram-negative bacteria (E . coli ) versus gram-positive bacteria (S . 金黄色葡萄球菌 和 S . epidermidis ) and yeast (C . albicans )。 The results showed that the decoration of GO with Ag-NPs promotes a synergistic effect and reduces dramatically the concentrations required to inhibit all tested bacterial and yeast strains. The antimicrobial potential of Ag-GO is also influenced by the thickness of the cell wall of the microorganisms. The thicker peptidoglycan layer in gram-positive bacteria, such as S . 金黄色葡萄球菌 和 S . epidermidis , may explain why these bacteria are more resistant to the antibacterial effects of GO-Ag. A disruption of membrane functionality from an interaction between released Ag nanoparticles/Ag + ions and the cell membrane and extensive cell membrane damage caused by the formation of ROS ultimately caused damage to the cell due to oxidative stress. In order to explain the mechanism of ROS production, additional studies are needed. Our research indicates the potential applicability of GO-Ag as an antimicrobial agent.
缩写
- 原子力显微镜:
-
原子力显微镜
- Ag-NPs:
-
银纳米粒子
- DLS:
-
动态光散射
- 开始:
-
氧化石墨烯
- GO-Ag:
-
Graphene oxide decorated with silver nanoparticles
- LDE:
-
Laser Doppler electrophoresis
- LDH:
-
Lactate dehydrogenase
- ROS:
-
活性氧
- SEM:
-
扫描电镜
- XRD:
-
X射线衍射
纳米材料
- 用贵金属纳米粒子装饰的电纺聚合物纳米纤维用于化学传感
- 钛酸盐纳米管装饰氧化石墨烯纳米复合材料:制备、阻燃和光降解
- 聚苯乙烯与掺杂十二烷基硫酸的聚苯胺的新型纳米复合材料
- 使用多功能 GaN/Fe 纳米颗粒靶向内皮细胞
- 石墨烯和氧化石墨烯的体外和体内生物安全和抗菌能力
- Ag 纳米颗粒/BiV1-xMoxO4 与增强的光催化活性的协同效应
- 磁性聚(N-异丙基丙烯酰胺)纳米复合材料:制备方法对抗菌性能的影响
- 用 6-巯基嘌呤和神经元穿透肽修饰的金纳米颗粒促进 SH-SY5Y 细胞生长
- 原位制备的壳聚糖/银纳米颗粒溶液对耐甲氧西林金黄色葡萄球菌菌株的抗菌活性
- 银纳米结构的合成方法和应用的最新进展
- PEG 包覆的 CoFe2O4 纳米颗粒的毒性与姜黄素的处理效果
- 石胆酸修饰的金纳米粒子对肝癌细胞的凋亡作用


